Many ocular disorders are characterized by abnormalities in the structure and function of various areas of the back of the eye, generally known as the “eye fundus.” Fluorescent pigments and small yellowish deposits called drusen, for example, build under the retina in age-related macular degeneration, while glaucoma is distinguished by the degeneration of neurons called ganglion cells.
 The proposed system can take detailed images of the eye fundus in real time while simultaneously measuring the spectral profile of a small target region, whose position can be readjusted easily without requiring patient fixation. These capabilities make it easier to observe the spectral properties of specific structures or lesions in the retina, which can help in the diagnosis of various conditions. Image Credit: Lapointe et al., doi 10.1117/1.JBO.28.12.126004
The proposed system can take detailed images of the eye fundus in real time while simultaneously measuring the spectral profile of a small target region, whose position can be readjusted easily without requiring patient fixation. These capabilities make it easier to observe the spectral properties of specific structures or lesions in the retina, which can help in the diagnosis of various conditions. Image Credit: Lapointe et al., doi 10.1117/1.JBO.28.12.126004
Interestingly, alterations in the fundus of the eye are not limited to vision-related illnesses. Certain neurological diseases, such as Parkinson’s and Alzheimer’s, can alter retinal neurons and blood flow.
To identify ocular diseases, eye care specialists often use computed tomography and color imaging methods. However, over the last several decades, researchers have shown that alterations caused by disease also alter the eye fundus’s spectral reflectance and emittance profiles.
Put differently, crucial diagnostic information can be obtained from the way light interacts with particular retinal structures at different frequencies in addition to normal imaging techniques.
Consequently, spectral analysis methods and instruments for light reflected or emitted by the fundus of the eye have been progressively gaining popularity. However, even though several approaches have been put out, they have significant drawbacks.
The inability of most spectroscopy-based techniques to measure across a small area of the eye fundus makes it difficult for them to identify minute spectral changes in small retinal structures, which is one prevalent problem. However, methods capable of producing localized spectral measurements need the patient’s fixation, which can be a laborious and difficult process.
Under the direction of Professor Dominic Sauvageau of the University of Alberta, a research team from Zilia Inc. in Canada has created a far more adaptable technology for targeted spectroscopy in the eye fundus to address these problems. Their study, which was published in the Journal of Biomedical Optics (JBO), explains the thinking behind their design and uses several in-depth tests to show off its possibilities.
A few distinguishing characteristics of the suggested system set it apart from the more flexible and adaptable options. Three distinct light routes to and from the eye fundus could effectively coexist without interfering with one another, thanks to various optical components across the whole device.
More precisely, continuous color imaging and spectral measurements can be obtained by utilizing illumination LEDs, a color camera, and a spectrometer all at the same time.
Moreover, the device’s spectrometer part concentrates an LED onto a small portion of the eye fundus, and it has the ability to spin the beam splitter that supplies the camera and spectrometer to change the location of this area. These actuators are simple mechanical devices.
The user can select a target and move it to any location within the eye fundus region being imaged without any realignment or change of the fixation target while continuously receiving spectral information of the targeted sampled area.
Dominic Sauvageau, Professor, University of Alberta
This capability facilitates the simple acquisition of spectral data from highly accurate anatomical features, including the retina, optic nerve, blood leaks, fat deposits, and lesion types. Notably, by modifying the light source, the system can also be used to do fluorescence measurements, expanding its range of applications for detecting biomarkers.
The study team conducted both in vitro and in vivo experiments to validate the functionality and performance of their system. In the in vitro tests, spectral measurements were made of an eye model that imitated a macula, blood vessels, a foreign substance, and the optic nerve by aiming colored portions of the target toward a reference target that had a grid-like pattern.
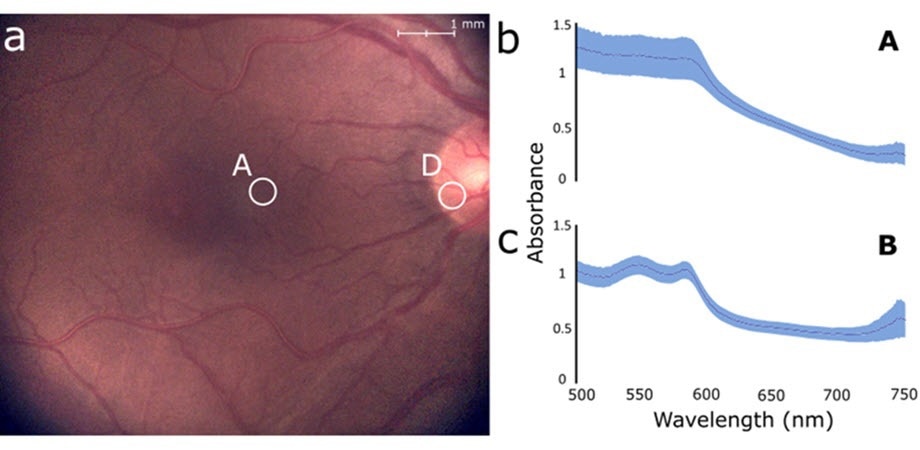
In contrast, eight healthy people’s retinas were used for the in vivo measurements, and the results showed variations in the spectral profiles of the optic nerve and the parafoveal area.
When combined, the study’s findings demonstrate the various benefits of the suggested design and could open the door to more effective methods of diagnosing eye conditions.
“Targeted ocular spectroscopy has the potential to assess the presence of different chromophores and fluorophores, such as hemoglobin, oxyhemoglobin, melanin, and lipofuscin, associated with disease progression. This could open the door to changes in the way we diagnose and treat eye diseases, and targeted ocular spectroscopy could become an increasingly important tool in eye care in the coming years,” Sauvageau added.
Journal Reference:
Lapointe, N., et. al. (2023) Targeted spectroscopy in the eye fundus. Journal of Biomedical Optics. doi:10.1117/1.JBO.28.12.126004.