Tau aggregates are a notable form of amyloid protein and hence have turned into a main focus of research for unveiling the mechanisms underlying neurodegeneration.
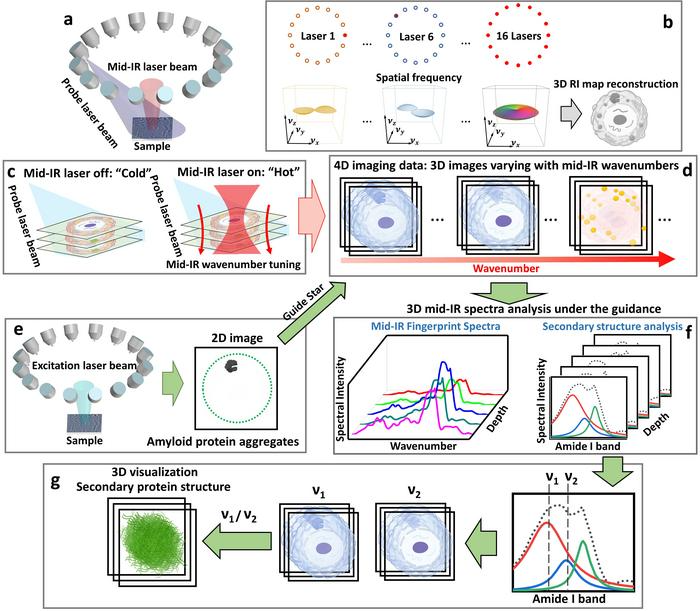
a Pump-probe 3D chemical imaging scheme. b 3D RI map reconstruction scheme. c “Cold” state: imaging without mid-IR pump beam; “Hot” state: imaging with mid-IR pump beam. d Sample reconstruction results. e 2D fluorescence intensity imaging. f Depth-resolved mid-IR fingerprint spectra generation and related protein secondary structure spectroscopic analysis. g 3D visualization of protein secondary structure. Image Credit: Jian Zhao, Lulu Jiang, Alex Matlock, Yihong Xu, Jiabei Zhu, Hongbo Zhu, Lei Tian, Benjamin Wolozin, and Ji-Xin Cheng
Several kinds of tau aggregates, such as tau oligomers and fibrils, have been utilized for an extensive range of neurodegenerative diseases. But the formation mechanism of tau aggregates and the linked disease pathways is yet to be understood clearly.
Analyzing tau aggregation requires high-resolution chemical imaging tools that have the potential to define intracellular volumetric tau aggregates inside their native surroundings.
Numerous methods have been developed for this cause, like Cryo-Electron Microscopy (Cryo-EM), X-Ray crystallography, circular dichroism spectroscopy, nuclear magnetic resonance spectroscopy, and atomic force microscopy-based infrared spectroscopy imaging.
Such methods have made considerable progress in specifying several kinds of amyloid proteins, such as tau aggregates. Still, the present solutions available are constrained by their inability to offer volumetric site-specific spectroscopic analysis and three-dimensional imaging of intracellular protein aggregates present in their native cellular states.
Hence, recovering three-dimensional chemical data of intracellular protein aggregates in their native cellular environments is considered to be a major hindrance.
In a recent publication in the journal Light Science & Applications, Dr. Ji-Xin Cheng from the Photonics Center at Boston University, Dr. Jian Zhao (former member of the Cheng Lab at Boston University) from the Picower Institute for Learning and Memory at the Massachusetts Institute of Technology, Dr. Benjamin Wolozin from the School of Medicine at Boston University, Dr. Lei Tian from the Department of Electrical and Computer Engineering at Boston University, and their collaborators presented a computational fluorescence-guided mid-infrared (mid-IR) photothermal microscope called Fluorescence-guided Bond-selective Intensity Diffraction Tomography (FBS-IDT).
FBS-IDT combines single-photon 2D fluorescence imaging into pump-probe pulsed MIP intensity diffraction tomography. This groundbreaking method enables molecular-specific 3D chemical imaging and site-specific mid-IR spectroscopic analysis of intracellular tau fibrils inside the cellular fluid.
Particularly, FBS-IDT allows the effective extraction of chemical information concerning particular protein aggregates from background protein signals inside cellular fluids. FBS-IDT’s 3D spectroscopic imaging has the potential to obtain high resolutions (~350 nm laterally, ~1.1 µm axially) and high speed (~0.05 Hz, up to ~6 Hz).
Leveraging this hyperspectral 3D chemical imaging capability, FBS-IDT illustrated the possible correlation between lipid accumulation and tau fibrils. Remarkably, this method has the potential of extracting depth-resolved mid-IR fingerprint spectra and envisioning the protein secondary structure of intracellular tau fibrils present in three-dimensional space.
The researchers stressed the importance and benefits of the FBS-IDT method:
Scientists stated, “FBS-IDT overcomes the challenges associated with 3D chemical imaging of intracellular amyloid protein aggregates and their protein secondary structures within fluid environments. This accomplishment is realized through a scan-free and modular design that utilizes a low-cost brightfield microscope with add-on light sources.
The investigators continued, “The cost of the FBS-IDT system is at least 30 times lower than that of state-of-the-art electron microscopy methods, such as Cryo-EM, and it incurs negligible operational fees. This cost-effective tabletop system is suitable for routine usage in most laboratory settings.”
“Our technique imposes no additional restrictions on biological samples, thereby opening up a new avenue for in vivo imaging of intracellular protein aggregates. Furthermore, our method is built on a modular design that can be readily adapted to fulfill diverse imaging requirements. We firmly believe that our FBS-IDT method can offer novel contributions to neurodegeneration research and various biomedical applications,” they added.
Journal Reference
Zhao, J., et al. (2023). Mid-infrared chemical imaging of intracellular tau fibrils using fluorescence-guided computational photothermal microscopy. Light: Science & Applications. doi.org/10.1038/s41377-023-01191-6