Infrared (IR) spectroscopy is the most widely used experimental material characterization and analysis technique.
Image Credit: boonchok/Shutterstock.com
An estimated 10 million organic chemicals are used in industrial production, while another 100,000 organic compounds are created yearly. Chemicals are either produced by chemists in laboratories or separated from natural materials. Scientists characterize each new compound and group them according to standardized protocols.
Many experimental methods have been developed to analyze chemical compounds. X-ray crystallography, Nuclear Magnetic Resonance (NMR), mass spectrometry and UV/visible and IR spectroscopy are some of the instrumental techniques available for analysis.
IR spectroscopy has emerged as one of the most popular experimental tools due to its capabilities in providing a broad range of structural information. IR spectroscopy is also less expensive to implement when compared with other methods.
IR Spectroscopy
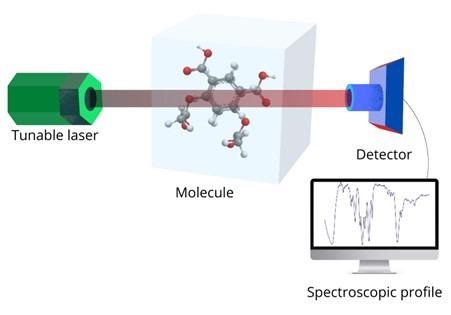
Figure 1: A typical experimental setup for IR spectroscopy. Image Credit: Ilamaran Sivarajah
IR spectroscopy refers to the interaction of the IR portion of electromagnetic radiation with matter. A typical experimental setup to implement IR spectroscopy is shown in figure 1.
First, a light source that can emit IR radiation is chosen. This can be a white light source with a monochromator or a tunable laser.
The radiation source should emit IR light that can produce sharp wavelength bands. For example, a tunable laser that can go from 2 micrometers to 5 micrometers in 0.1-micrometer steps.
The IR light is then directed at the material that is being studied. The incident IR radiation alters the molecules in the sample. The atoms and chemical bonds that make up the molecules can absorb or shift the frequency of light. A detector then collects the IR radiation that has passed through the material and a spectral profile is produced.
Structural information of the sample can be inferred by analyzing the spectral data and comparing it to previously known spectral profiles.
Distinguishing Functional Groups
Molecules are composed of atoms and atomic bonds. When a group of atoms within a molecule exhibit similar chemical properties, they are defined as belonging to a particular functional group. These atoms maintain their properties when they are in different chemical compounds. Even in molecules composed of many different atomic bonds, the behavior of a given functional group can be isolated.
The electromagnetic field of IR light causes oscillations within the molecule. As a result, molecular bonds vibrate at different frequencies. Constituents of a particular functional group have characteristic vibrational properties.
The unique features depend on the atoms, the type and number of bonds, and the orientation of the bonds with respect to the rest of the molecule. By acquiring the IR spectra, it is possible to correlate the vibrational bond frequency. The type compound can be distinguished from bulk material.
Identifying Molecular Structure
IR spectroscopy identifies unknown organic materials. Spectroscopic profiles of the unknown samples are compared with that of known compounds.
An extensive database of IR spectra of organic molecules has been documented over a long time.
Known as molecular fingerprinting, the structure of many molecules has been encoded into a repository that can be retrieved for comparison. Fingerprint data typically shows the presence or absence of particular compounds in a molecule.
Detecting Hazardous Materials
IR spectroscopy has been successfully used to detect hydrocarbons and hazardous chemicals remotely. For example, IR spectroscopy can be performed in the region surrounding the leak when a gas leak is identified. The spectroscopic profile collected at the scene is matched to the existing details of harmful molecular agents. This enables quick and targeted clean-up of the compromised area.
Progress of Chemical Reactions
In the process of material production, various chemical reactions take place. During such reactions, many intermediate compounds are formed. Depending on the production process, intermediate materials are also used for manufacturing. IR spectroscopy is a useful diagnostic tool to quality control the manufacturing process. A regular interrogation of the reactionary process identifies the compounds and reactants.
Fundamental Dynamics of Matter
IR spectroscopy, complemented by mass spectrometry, is used to study the formation of matter. IR radiation is often used to photo detach molecules to investigate the conditions they are formed. These types of experiments are sometimes conducted in cryogenic environments to mimic the conditions in space.
Photonics
Apart from the immense benefits of IR spectroscopy in chemistry, the photonics industry has also gained much use. The optical communication infrastructure is operated in the near IR region of the electromagnetic spectrum. Optical elements such as fibers, waveguides, optical modulators and many other components are needed for an optical network to function. New and more efficient optical components are regularly developed. IR spectroscopy is an invaluable tool in the characterization of optical components.
Outlook
The enormous advantages of IR spectroscopy are already well established. Many different aspects of the IR spectroscopy setups are continuously developed and advanced.
Better optical sources and more sensitive detectors will enhance spectroscopic details. The conditions and the environment under which materials are studied are also explored in depth.
References and Further Reading
Thompson, J.M. (2018). Infrared Spectroscopy (1st ed.). Jenny Stanford Publishing. https://doi.org/10.1201/9781351206037
Seo, M., Shin, H.K., Myung, Y. et al. Development of Natural Compound Molecular Fingerprint (NC-MFP) with the Dictionary of Natural Products (DNP) for natural product-based drug development. J Cheminform 12, 6 (2020). https://doi.org/10.1186/s13321-020-0410-3
Celio Pasquini, Near infrared spectroscopy: A mature analytical technique with new perspectives – A review, Analytica Chimica Acta, Volume 1026, 2018, Pages 8-36, ISSN 0003-2670, https://doi.org/10.1016/j.aca.2018.04.004.
Yves Roggo, Pascal Chalus, Lene Maurer, Carmen Lema-Martinez, Aurélie Edmond, Nadine Jent, A review of near infrared spectroscopy and chemometrics in pharmaceutical technologies, Journal of Pharmaceutical and Biomedical Analysis, Volume 44, Issue 3, 2007, Pages 683-700, ISSN 0731-7085, https://doi.org/10.1016/j.jpba.2007.03.023.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.