A new method, depending on laser ablation, has been developed by researchers to perform biopsies quicker, more affordably, and in a way less detrimental to the patient.
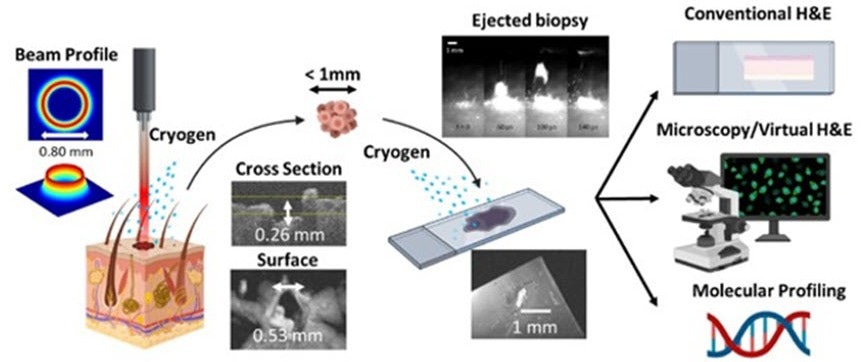
Overview of the novel laser microbiopsy system, which is fast and minimally invasive. A laser is focused onto the target tissue, causing a small volume of it to be ejected. The tissue sample is collected using a glass slide and can be analyzed via various methods. A cryogenic spray is applied at the target area and the glass slide to cool down the sample and prevent thermal damage. Credit: King et al., doi.org/10.1117/1.JBO.27.12.125001
Biopsies are known to be one of the most extensively utilized diagnostic procedures for several kinds of diseases, including cancer. Currently, the gold standard is to mechanically withdraw a tissue section, further cut it into thin slices, and stain these with hematoxylin and eosin (H&E) to make the nuclei and cytoplasm of cells simpler to see with a microscope.
While this method is well-fixed in clinical practice, it has several drawbacks that call for the growth of alternative procedures.
In a recent study reported in the Journal of Biomedical Optics, a research group headed by James W. Tunnell of The University of Texas at Austin, and Thomas E. Milner of the University of California Irvine has come up with a revolutionary laser-based method to execute microbiopsies.
Their technique involves the usage of a concentrated laser beam to expel a small volume of cells obtained from the irradiated tissue, gather them onto a glass slide, and utilize a staining procedure known as virtual H&E.
The traditional pathology workflow suffers from limitations, including biopsy invasiveness and complex and time-consuming tissue processing; our protocol based on laser microbiopsy with virtual H&E imaging shows promise as a rapid and minimally invasive tool for biopsy and diagnosis.
Jason B. King, Study First Author, The University of Texas at Austin
The primary mechanism at play in this novel microbiopsy method is laser ablation. This is a phenomenon in which the concentrated energy of laser pulses has been absorbed by the target across a small superficial volume, thereby resulting in a matter rapidly heating up and being expelled.
Even though laser ablation is more extensively utilized in machining and materials manufacturing, the researchers sought to make use of this principle in a way that is consistent with tissue extraction and analysis.
To obtain this, the team had to defeat various hindrances. Initially, they had to develop the optical setup and improve the energy and profile of the laser beam. Utilizing just commercially available equipment and an FDA-approved Ho:YAG lasers, the scientists constructed a tabletop laser microbiopsy system that would allow comfortable use in a clinical setting.
Following careful analysis of the characteristics of the beam profile in a theoretical manner and via experiments, they executed complete computational simulations of the full laser microbiopsy process.
This offered a useful understanding of how the laser beam could communicate with tissue and enabled them to choose the best parameters before proceeding to actual experiments.
As soon as a prototype was all set and configured, the scientists made use of the system to extract extremely low volumes of tissue (less than the microliter range) from porcine skin and kidneys. They stained such tissue samples for virtual H&E and examined them with a confocal microscope.
One significant idea for the suggested protocol is that scientists can spray both the target tissue area and the glass slide to restrict the extent of thermal damage that has been caused as a result of the ablation process. This has proved to be necessary to achieve good-quality samples.
The total outcomes of the experiments are highly promising, as the majority of the sampled tissue was liberated from thermal damage. The volumes that were extracted were fairly low to be minimally invasive, yet enough for virtual H&E analysis.
This implies that a patient could experience dozens of laser microbiopsies without causing nearly as much damage as standard biopsies.
One more exceptional benefit is that the entire tissue extraction and virtual H&E analysis protocol could be done in just a few minutes, rather than half an hour or even days for standard biopsies with traditional tissue processing.
Journal Reference:
King, J. B., et al. (2022) Tissue harvest with a laser microbiopsy. Journal of Biomedical Optics. doi.org/10.1117/1.JBO.27.12.125001.