Jun 17 2020
Multicolor fluorescence microscopy is a crucial tool in the field of life sciences to analyze the relative arrangements of cellular structures or interactions of different proteins.
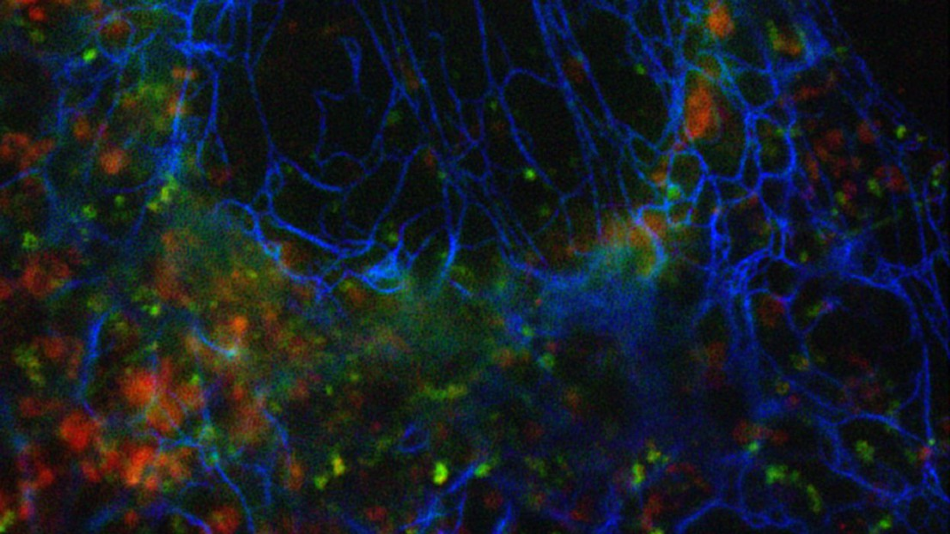
Image Credit: © 2020 EPFL.
But traditional microscopes, which are the workhorses for various biological studies, have the ability to resolve details only of the order of the wavelength of light. In the last 20 years, various super-resolution microscopy approaches enabled researchers to overcome this diffraction limit and to achieve new discoveries.
These new techniques are gradually being used for regular biological applications. For a few of the new techniques, this is not just due to complex microscope hardware but also significant hurdles posed by more and more demands on sample preparation and fluorescent labels. The demands for successful super-resolution imaging are ever more difficult to fulfill for multicolor applications.
Led by Theo Lasser, the Laboratory of Biomedical Optics at EPFL has been working extensively on Super-resolution Optical Fluctuation Imaging (SOFI) to enhance spatial resolution and sampling in 2D and 3D. SOFI is a substitute for Single-molecule Localization Microscopy techniques like PALM and STORM.
It involves analyzing higher-order spatio-temporal statistics of a time-series of blinking fluorophores and avoids the need to isolate emissions of individual fluorophores. SOFI is adaptable with an extensive range of imaging and labeling conditions, thereby simplifying fluorophore selection and experiments.
As part of the new study, led by Theo Lasser and Aleksandra Radenovic (head of the Laboratory of Nanoscale Biology), scientists at the School of Engineering extended the statistical analysis into the spectral domain to develop a new method for multicolor super-resolution imaging.
The number of colors is not restricted by the spectral channels of the microscope or exploiting spectral crosstalk.
The concept based on which the multicolor SOFI method works is as follows. Classical multicolor imaging requires the removal of crosstalk between various spectral channels of the microscope. In this method, the crosstalk is exploited to produce additional color channels. Cross-cumulant analysis is applied between various spectral channels acquired simultaneously.
The statistical analysis enables them to augment the physical detection channels offered by the microscope with more virtual spectral channels.
Only the signals that are spatially and temporally correlated in the different spectral channels will appear in the virtual channels. We are picking up exactly the crosstalk that everyone else wants to get rid of.
Kristin Grußmayer, Study Lead Authors, EPFL
In combination with linear unmixing, the additional computationally generated spectral channels enable the imaging of more unique fluorophore colors compared to the physical detection channels that were recorded.
The study describes the theory underlying spectral cross-cumulant multicolor SOFI and offers a framework to improve the microscope’s spectral channels for a specified combination of fluorophores to be imaged.
Using simulated datasets, the researchers were able to confirm that their new multicolor method would apply for an extensive range of labels with various photophysical properties, even for those that have strongly overlapping emission spectra.
We could show that our approach works for three color imaging in fixed and in living cells for a variety of dyes and fluorescent proteins. The imaging can be performed using commercial widefield setups with two-channel image splitting units that are widely available. In principle, we are not limited to 3 colors.
Kristin Grußmayer, Study Lead Authors, EPFL
Funding
This study was financially supported by EPFL, NVIDIA Corporation GPU grant, and European Union’s Horizon 2020 research and innovation program Marie Skłodowska-Curie Grant Agreement No. [750528] for K.S.G.
Journal Reference:
Grußmayer, K. S., et al. (2020) Spectral cross-cumulants for multicolor super-resolved SOFI imaging. Nature Communications. doi.org/10.1038/s41467-020-16841-1.