Apr 9 2020
Through a combination of computational and experimental data, scientists have now identified ways to improve the pulses emitted by extremely powerful X-ray beams.
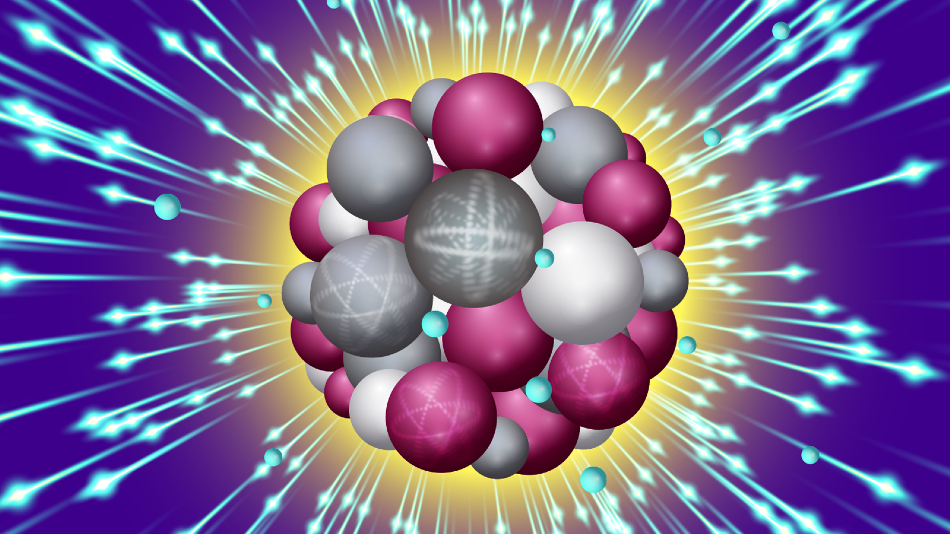 An intense X-ray pulse scatters off a sucrose cluster (red, white, and gray spheres are oxygen, carbon and hydrogen atoms, respectively) resulting in ejected electrons (blue spheres) and structural deformation. Image Credit: Stacy Huang.
An intense X-ray pulse scatters off a sucrose cluster (red, white, and gray spheres are oxygen, carbon and hydrogen atoms, respectively) resulting in ejected electrons (blue spheres) and structural deformation. Image Credit: Stacy Huang.
For a long time, researchers have been exploring the ability to observe the structure of a single, free-form molecule at atomic resolution, what is called the “holy grail” of imaging by many scientists.
In one promising technique, highly intense, extremely short, X-ray free-electron laser (XFEL) pulses are aimed at a sample material. However, this ultrafast imaging method also tends to damage its target, and hence time is very important.
At the Argonne National Laboratory of the U.S. Department of Energy (DOE), scientists are improving the effort by using a combination of computer simulations and experiments, in an effort to understand how interactions occur between the XFEL pulses and their targets.
Recently, a research team, headed by Atomic Molecular Optical Physics group in the Chemical Sciences and Engineering division in Argonne National Laboratory, pointed out that time is a vital and commonly ignored parameter that can have an impact on the experiment results.
The researchers’ study, titled “The role of transient resonances for ultra-fast imaging of single sucrose nanoclusters,” was recently reported in the Nature Communications journal.
For instance, the potential to analyze three-dimensional (3D) structures at the atomic scale can improve one’s understanding of viruses, and thus help deliver drugs to the body in a more effective manner.
Currently, for this kind of analysis, the material to be investigated should be put in a crystalline form. This non-native form is fixed with biological particles so that when an X-ray strikes these particles, the beam scatters and produces a diffraction pattern that can be utilized to interpret the molecular structure.
However, there are several types of biological systems that do not crystallize very well, and the crystals may also be very small to produce an excellent diffraction pattern. The structure could even be changed by crystallization, thus inhibiting the potential to view a particle in its natural state.
However, to produce a scatter pattern without crystallizing the material, a highly powerful beam, such as XFEL, is required. This beam is flashed in rapid bursts that are mind-boggling.
For this type of experiment, you need very intense pulses, which can destroy the sample very quickly. With this approach, you need to use very short pulses so you can collect all the scattering signals before the sample is destroyed.
Phay Ho, Study Co-Author and Physicist, Argonne National Laboratory
This race against time is quantified in femtoseconds, one of which corresponds to a millionth of a billionth of a second.
To investigate how different parameters can have an impact on the outcome of an XFEL experiment, the cross-disciplinary research team probed single nanoclusters of sucrose utilizing the Linac Coherent Light Source (LCLS), an XFEL deployed at SLAC National Accelerator Laboratory in Stanford University.
The crystals that you observe at a storage-ring based light source such as Argonne’s Advanced Photon Source (APS), as opposed to an XFEL, are typically 10 microns or so in size. The structures we are looking at in this study are at least 200 times smaller—nanometers in size.
Linda Young, Study Co-Author and Distinguished Fellow, Argonne National Laboratory
The experimental data were then compared with the calculations run on the supercomputer Mira, installed at the Argonne Leadership Computing Facility (ALCF). This included a massive ensemble of molecular simulations that monitored nearly 42 million particles communicating with a single FEL pulse—a task for a supercomputer.
“When you have a machine like Mira, you can run a large number of simulations, you can do them all at the same time, and you can run them over the timescales that we needed for this particular study,” stated Christopher Knight, the study’s co-author and a computational scientist with the ALCF and Argonne National Laboratory’s Computational Science division.
The study also discovered that the shorter is better when it comes to XFEL pulses on sucrose. Researchers looking to increase the imaging outcomes could utilize a pulse length of 200 femtoseconds. However, it was found that 200 millionths of a billionth of a second may be too easy.
If you use pulses this long, you can actually degrade your signal substantially. In order to do this type of imaging, the pulse should last only a few femtoseconds. It’s important to look not just at the number of photons, but the number of photons per unit of time.
Phay Ho, Study Co-Author and Physicist, Argonne National Laboratory
The computer modeling will aid the scientists to improve upcoming experiments and thus zero in on the parameters that will generate the most optimal results.
“It’s not easy to get the beam time to do these experiments,” added Ho. “This data will be very useful in figuring out the optimal pulse conditions to try next.”
The LCLS, the APS, and the ALCF are DOE Office of Science User Facilities.
The study was finally supported by the Swedish Foundation for International Cooperation in Research and Higher Education (STINT), the Swedish Research Council, DOE’s Office of Basic Energy Sciences, the Knut and Alice Wallenberg Foundation, the Peter Paul Ewald Fellowship from the Volkswagen Foundation, the Swedish Foundation for Strategic Research, and the European Research Council.
Computing time at the ALCF was awarded via DOE’s ASCR Leadership Computing Challenge.