Jun 24 2016
The medical field of the future is most likely to include wide-ranging tissue-engineering technologies such as organoids (tiny organs grown from stem cells) and organs-on-chips. However this prediction is made based on a simple yet demanding task – manipulating cellular performance in three dimensions. As of now, a majority of cell culture methods are restricted to 2D environments such as a chip or a Petri dish, but that does not match real biology or assist researchers in sculpting organs and tissues.
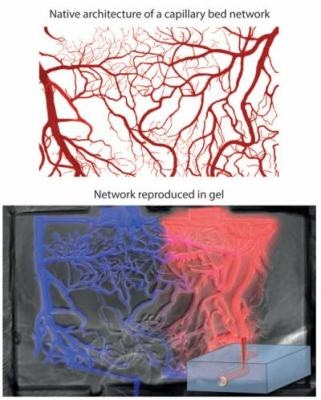 A diagram showing how the architecture of a capillary bed can be reproduced in a 3D cell culture hydrogel using short-pulse lasers. (Photo credit: Matthias Lütolf/EPFL)
A diagram showing how the architecture of a capillary bed can be reproduced in a 3D cell culture hydrogel using short-pulse lasers. (Photo credit: Matthias Lütolf/EPFL)
A unique technique has been developed by two EPFL researchers. It involves the application of lasers to cut out paths within biocompatible gels to locally manipulate cell operation and support tissue formation.
Their research findings have been published in Advanced Materials.
Cells grow in 3D microspaces in the body, and they are specific to each type of tissue e.g., heart, lung, brain, kidney, and others. These microenvironments are vital as they manage the behavior of the cells, e.g. the way in which they cooperate with other tissue parts to help it progress, function, and repair.
Furthermore, the microenvironments are very adaptable and dynamic themselves, transmitting a variety of biochemical signals to the cells to adapt their behavior to physiological modifications. This indicates that any victorious integration of engineering and biology has to primarily be able to grow cells in custom-designed yet biologically dynamic 3D spaces.
Matthias Lütolf and his PhD student Nathalie Brandenberg working out of EPFL’s Institute of Bioengineering, have formulated a technique that utilizes a laser to carve 3D networks and pathways for cells within a hydrogel scaffold that complies with their natural environment.
The technique integrates lasers with microfluidics, which is the science of manipulating fluids in micrometer-sized spaces. In this, the researchers applied focalized short-pulsed lasers, which can produce adequate power to develop miniature tunnels in a variety of biocompatible gels already used in tissue engineering and cell biology. It is possible to use the laser prior to or even during 3D cell culture, signifying that the cells can be manipulated in real time to conform to their natural growth.
In the interim, microfluidics has become the crucial aspect in tissue engineering. The technology provides unparalleled control over the microenvironment of cells, as it can copy the intricate adaptability of biological microenvironments, thus enabling behavior-modifying signals to be supplied to the cells in drug or other compound form.
Microfluidics is widely used to construct cell culture systems for growing cells. But microfluidics has so far been mostly restricted to 2D cell-culture applications, and is not simple to use in long-term cultures.
Using microfluidics in 3D cultures in certain cases has been quite positive; however they require a number of labor-demanding steps that cause them to be inefficient for regulated applications. Researchers Brandenberg and Lütolf have brought simplicity, strength and flexibility to the approach by integrating microfluidics with the versatlity of laser carving.
Our method addresses the limitations of previous approaches. It is fully compatible with 3D cell cultures, and can be applied with a wide range of materials, different geometries, and can introduce or change existing microfluidic networks during the course of an experiment to control cells in an unprecedented way.
Lütolf
The EU framework 7 HEALTH research programme PluriMes and an ERC grant funded this research.