Knowing how a living cell works means knowing how the chemistry inside the cell changes as the functions of the cell change. Protein phosphorylation, for example, controls everything from cell proliferation to differentiation to metabolism to signaling, and even programmed cell death (apoptosis), in cells from bacteria to humans. It’s a chemical process that has long been intensively studied, not least in hopes of treating or eliminating a wide range of diseases. But until now the close-up view – watching phosphorylation work at the molecular level as individual cells change over time – has been impossible without damaging the cells or interfering with the very processes that are being examined.
 Berkeley Lab scientists imaged individual cells as they were stimulated by nerve growth factor and simultaneously obtained absorption spectra using synchrotron radiation from the Advanced Light Source.
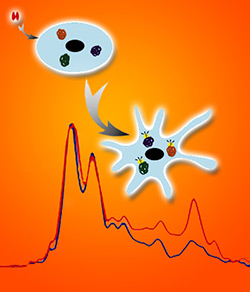
Berkeley Lab scientists imaged individual cells as they were stimulated by nerve growth factor and simultaneously obtained absorption spectra using synchrotron radiation from the Advanced Light Source.
“To look into phosphorylation, researchers have labeled specific phosphorylated proteins with antibodies that carry fluorescent dyes,” says Hoi-Ying Holman of the U.S. Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab). “That gives you a great image, but you have to know exactly what to label before you can even begin.”
Holman and her coworkers worked with colleagues from the San Diego and Berkeley campuses of the University of California to develop a new technique for monitoring protein phosphorylation inside single living cells, tracking them over a week’s time as they underwent a series of major changes.
“Now we can follow cellular chemical changes without preconceived notions of what they might be,” says Holman, a pioneer in infrared (IR) studies of living cells who is director of the Berkeley Synchrotron Infrared Structural Biology program at Berkeley Lab’s Advanced Light Source (ALS) and head of the Chemical Ecology Research group in the Earth Sciences Division . “We’ve monitored unlabeled living cells by studying the nonperturbing absorption of a wide spectrum of bright synchrotron infrared radiation from the ALS.”
The researchers report their results in the American Chemical Society journal Analytical Chemistry.