Cryogenic electron microscopy (cryo-EM) is a powerful tool to image battery materials. Cryo-EM has provided new insights into designing batteries that are more efficient in storing energy.

Image Credit: Roman Zaiets/Shutterstock.com
Batteries play an important role as energy storage devices for a clean energy future. The electrification of personal and other modes of transportation is expected to significantly decarbonize the transport sector.
Electric vehicles can considerably reduce carbon emissions. The most widely used batteries in the auto industry are lithium-ion batteries (Li-ion). But the main barrier for their broad adaptation is the limitation to their driving range. This is due to the relatively low-energy density of the Li-ion battery.
To improve the energy density of Li-ion batteries, new high-energy-density battery chemistry must be investigated. The increased capacity will increase the distance an electric car can drive with a fully charged battery. This will also bring down the cost per Kilowatt Hour (kW.h).
Basics of a Lithium-Ion Battery
Battery chemistry is studied by experimenting with the materials that make up the battery. A lithium-ion battery stores lithium ions between an anode, which is the negative electrode and a cathode, the positive electrode (see figure 1). By shuttling the lithium ions between the electrodes, electrical energy can be stored and retrieved.
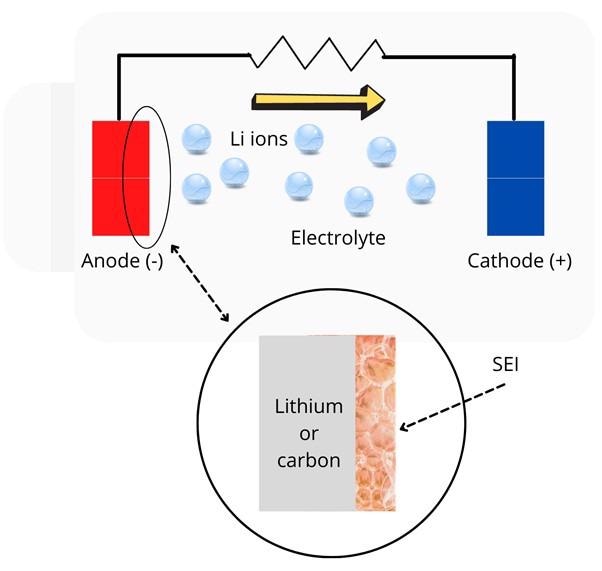
Figure 1: Illustration of a typical battery. Inset shows the solid electrolyte interphase (SEI). Image Credit: Ilamaran Sivarajah
The anode of lithium-ion batteries is commonly made of graphite. Six carbon atoms in a graphite lattice hold one lithium-ion. This ratio contributes to the low energy density in Li-ion.
To increase the energy density of the battery, more lithium ions must be introduced to the anode. An efficient method to improve the ion-to-anode compound ratio that has been explored is to replace graphite with elemental lithium or lithium metal. Ten times more elemental lithium can be packed in the same space as graphite. The excess elemental lithium compared to graphite improves the kW.h per kg from 300 to about 500, a vast improvement in the energy capacity.
While replacing graphite with elemental lithium presents an appealing innovation, it still cannot be implemented in battery production. This is due to the lack of understanding of the lithium chemical reaction and its dependence on the solid electrolyte interphase (SEI). SEI is an important structure within the battery.
Solid Electrolyte Interphase (SEI)
SEI is a surface layer that exists on every battery anode, whether it is graphite or Li metal. SEI reduces the liquid electrolytes within the battery. The reduction of liquid electrolytes forms a passivation layer on the anode that prevents further decomposition of the electrolytes. The SEI stabilizes the anode interface and dictates the ion transfer from the anode to the electrolytes. The process of ion transfer from the anode is critical to the performance of the battery.
To build a better battery, the structure and chemistry of the SEI must be better understood.
Methods for Studying SEI
Various experimental methods have been used to study SEI. For example, X-ray photoelectron spectroscopy (XPS) is a technique used to probe the SEI properties. This technique enables the formulation of the first SEI model. The molecular constituents of the SEI were shown to be made up of inorganic lithium-containing compounds such as oxides, carbonates, and fluorides close to the anode surface and more organic compounds such as organic carbonates closer to the electrolytes.
While XPS provided good chemical and depth information, it was limited in gathering in-plane resolution of the SEI structure. Understanding the distribution of SEI components on a cross-sectional plane requires a higher resolution probe technique.
Tunneling Electron Microscopy (TEM) captures images of chemical structures with nanoscale resolution. Traditional TEMs use high-energy electron beams to probe the samples. However, high-energy beams can damage samples such as battery materials.
Lithium compounds are usually very weakly bonded and are also highly reactive with air. When samples containing lithium are held in a TEM holder, they rapidly react with air and water vapor and easily corrode. The oxide deposited due to corrosion interferes with high-resolution imaging.
Cryogenic Electron Microscopy (cryo-EM)
To overcome the impediment of high reactivity, cryogenic electron microscopy (cryo-EM) has recently been explored. Recognized for its achievements with the 2017 Nobel prize in chemistry, cryo-EM enables in-depth, high-resolution analysis of soft samples shielded from reactive perturbations.
In cryo-EM, the sample under investigation is frozen to cryogenic temperatures using liquid nitrogen or ethane. The cooling process is carried out very rapidly so that samples are held in their natural form with a thin layer of ice protecting them from the outside environment. Electrons are fired at the sample and detected with a camera to produce an image.
The molecules in the sample are in different orientations. The application of a computer program sorts the images and groups all the molecules that are in the same orientation. Thousands of images are acquired for each sample. Additional software manipulation pieces the images together to produce a high-resolution 3D structure of the sample.
Being able to visualize matter with very minute detail allows one to better understand their dynamics. First high-resolution planar images of lithium-based anodes were taken with cryo-EMs. The distribution of compounds in the SEI has also been imaged using cryo-EMs with high accuracy.
Lithium fluoride, one of the compounds in the SEI, is critical for the performance of the battery. Further investigations using cryo-EMs are expected to allow scientists to control the amount of fluorine added to the battery material.
Future Outlook
Cryo-EM has been applied successfully for battery materials and interfaces to achieve impactful results in recent years. Better knowledge of nanomaterials has facilitated better battery development. Many unresolved questions about the battery materials and dynamics remain, for example, the soluble polysulfides and the structural nature of the oxidized oxygen anion at high voltages. Further advancements of cryo-EM and the development of complementary devices such as faster cameras can improve battery technology further.
References and Further Reading
Diyi Cheng, Bingyu Lu, Ganesh Raghavendran, Minghao Zhang, Ying Shirley Meng, Leveraging cryogenic electron microscopy for advancing battery design, Matter, Volume 5, Issue 1, 2022, Pages 26-42, ISSN 2590-2385, https://doi.org/10.1016/j.matt.2021.11.019.
Zhang, Z., Cui, Y., Vila, R., Li, Y., Zhang, W., Zhou, W., Chiu, W., and Cui, Y. (2021c). Cryogenic electron microscopy for energy materials. Acc. Chem. Res. 54, 3505– 3517. https://doi.org/10.1021/acs.accounts.1c00183
Zhang, B., Shi, H., Ju, Z., Huang, K., Lian, C., Wang, Y., Sheng, O., Zheng, J., Nai, J., Liu, T., et al. (2020a). Arrayed silk fibroin for high-performance Li metal batteries and atomic interface structure revealed by cryo-TEM. J. Mater. Chem. A. 8, 26045–26054. https://doi.org/10.1039/d0ta09753e
Xu, Y., Wu, H., Jia, H., Zhang, J.-G., Xu, W., and Wang, C. (2020b). Current density regulated atomic to nanoscale process on Li deposition and solid electrolyte interphase revealed by cryogenic transmission electron microscopy. ACS Nano 14, 8766–8775. https://pubs.acs.org/doi/10.1021/acsnano.0c03344
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.