AZoOptics features a new interview with Rebecca Spiecker of the Karlsruhe Institute of Technology, discussing the development of a new X-ray technique that allows better imaging and analysis of living organisms.
Please could you introduce yourself and your background in imaging research?
My name is Rebecca Spiecker, and I’m a Ph.D. student in the group of Professor Tilo Baumbach at the Karlsruhe Institute of Technology. During my studies, I worked on light microscopy at the Max-Planck-Institute in Göttingen under Professor Stefan Hell and on phase contrast techniques for electron microscopy in the group of Professor Dagmar Gerthsen at KIT. I was interested in imaging living samples and therefore switched to the field of X-ray phase contrast imaging for my Ph.D..
What motivated you and your team to develop a new X-ray imaging technique?
The concept of Bragg magnifiers had already been developed in the 70s; however, it has not found widespread usage yet. A challenge with imaging living samples is the high radiation damage that occurs during the imaging process. Our motivation was to tackle this challenge by optimizing Bragg magnifiers for high dose efficiency, minimizing radiation damage. This allowed us to extend imaging durations for living biological samples.
The researchers used the new technique to image tiny parasitoid wasps emerging from their host eggs. Even after 30 minutes of imaging, the wasps did not show any abnormalities in their behavior thanks to the minimal radiation exposure. Image Credit: Rebecca Spiecker, Karlsruhe Institute of Technology
What are the main challenges in traditional X-ray imaging methods that your new technique addresses?
Indirect detectors that are conventionally used in X-ray imaging consist of a scintillator, which converts the X-rays into visible light, and the image is then magnified by a microscope and detected by a CCD or CMOS camera. Because of the limited focal depth of the objective, the scintillator has to be very thin to reach high resolution. Due to the low thickness only a fraction of the X-ray photons is absorbed by the scintillator, leading to a low detection efficiency, especially at high X-ray energies. Consequently, a high X-ray dose is needed to attain a certain image quality and resolution, leading to radiation damage in the sample.
Could you provide a technical overview of your method and how you overcame these challenges?
The aim is to detect the X-ray photons efficiently. This can be done by highly efficient single-photon-counting detectors. However, single-photon-counting detectors have a rather large pixel size of in our case 55 microns. To attain micrometer resolution, the idea is to magnify the X-ray beam in front of the sample by using asymmetrically cut silicon crystals.
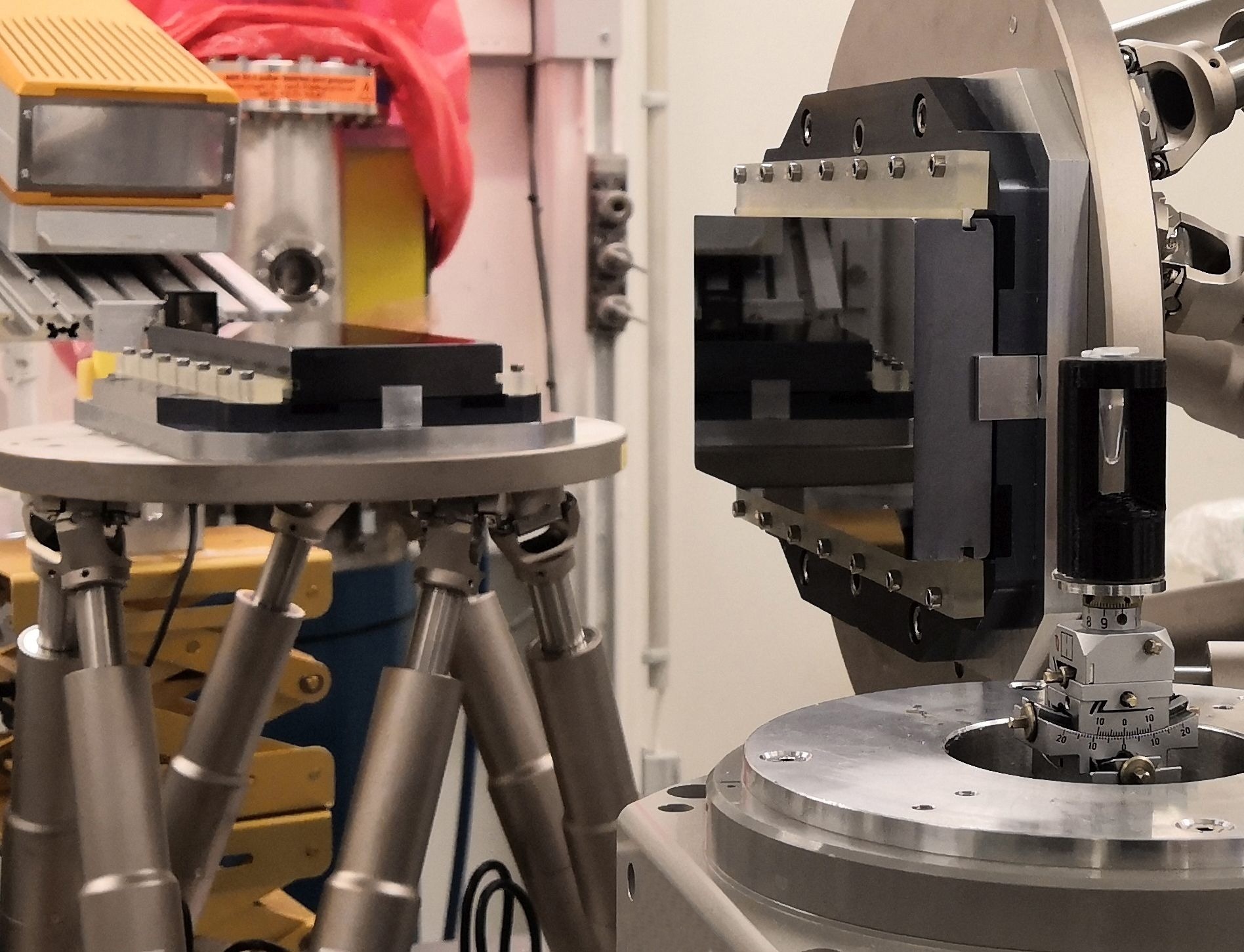
The new X-ray imaging technique uses a much lower X-ray dose thanks to two Bragg magnifier crystals (center) and a single-photon-counting detector (on the left). The sample is shown on the right. Image Credit: Rebecca Spiecker, Karlsruhe Institute of Technology
Could you explain the role of phase contrast imaging and Bragg magnifiers in your approach?
Conventional absorption imaging exploits the absorption of X-rays in the sample. Biological samples consist of light elements and have, therefore, very low absorption. On the other hand, the sample also imprints a phase shift on the incident X-ray wavefield, which is over two orders of magnitude higher than the absorption.
There exist different methods to make this phase shift visible. We use propagation-based phase contrast, one of the main approaches. By self-interference, the phase shift in the wavefield increasingly evolves into intensity contrast as the distance between the sample and the detector increases. To detect this phase contrast image with an efficient single-photon-counting detector we magnify the wavefield by a so-called Bragg magnifier.
A Bragg magnifier consists of perfect silicon crystals with an asymmetry angle, which means that there is an angle between the lattice planes and the crystal surface. The X-ray beam behind the sample is diffracted at the lattice planes by Bragg diffraction. Simultaneously, due to the asymmetry angle, the outgoing angle is increased with respect to the crystal surface. We magnify the beam in two dimensions by a factor of up to 150 using two crystals. In this way, we can exploit the high detection efficiency of single-photon-counting detectors while at the same time attaining micrometer resolution.
How does reducing the X-ray dose impact the study of living organisms, and what opportunities does this open up?
By reducing the dose, we can observe living samples for longer periods than currently possible before the onset of radiation damage, providing new insights into dynamical biological processes.
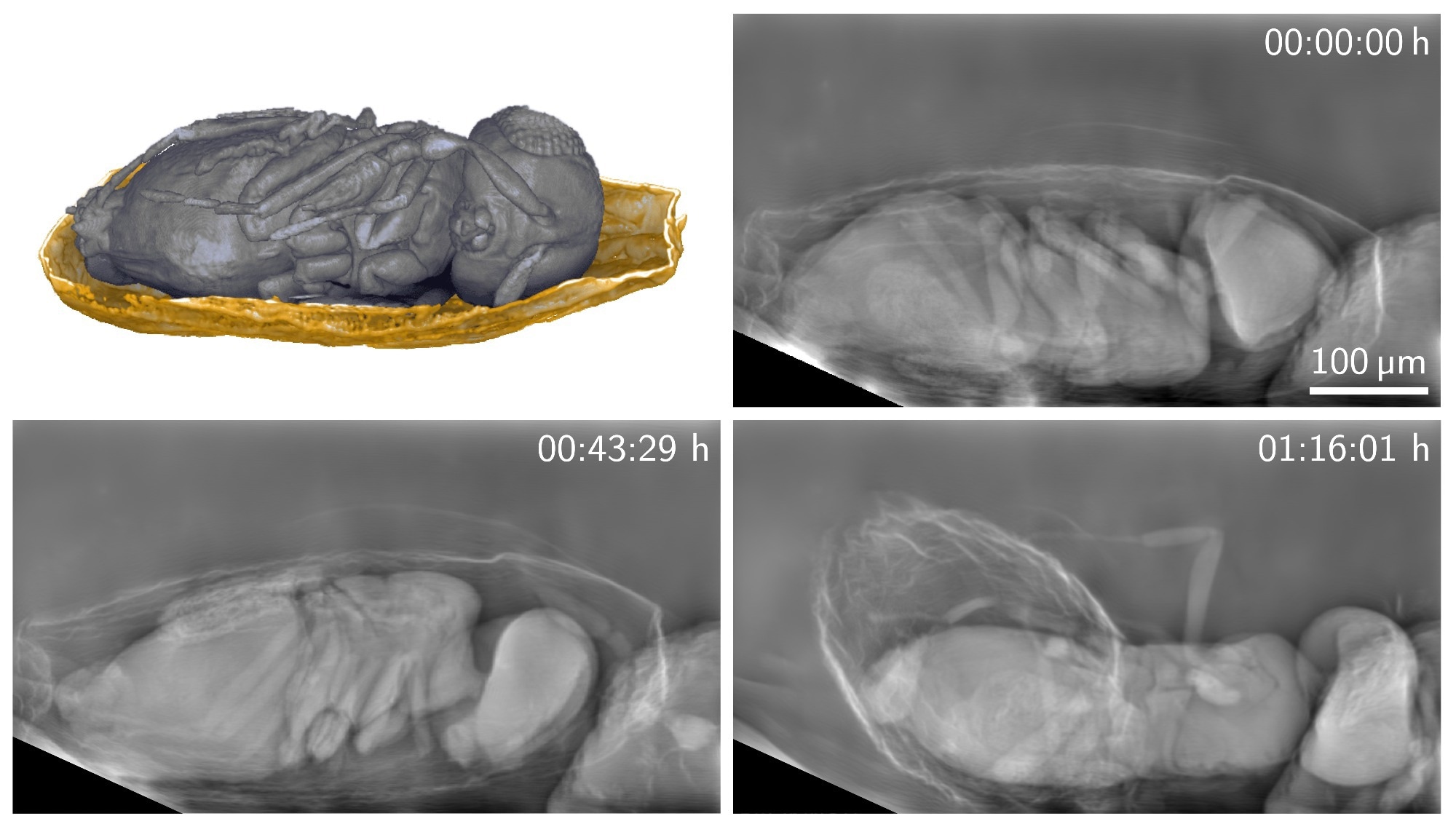
A new X-ray imaging technique can produce detailed images of living organisms with a much lower X-ray dose than previously possible. The researchers used the new technique to image tiny parasitoid wasps emerging from their host eggs for more than 30 minutes. Image Credit: Rebecca Spiecker, Karlsruhe Institute of Technology
Your study involved imaging parasitoid wasps over an extended period. What insights did this provide into their behavior or development?
So far, the behavior of parasitoid Trichogramma wasps during emergence from their host eggs has not been studied in vivo yet. We were now able to image their behavior before and during emergence for more than 30 minutes. We could observe the flexible biting capability of their mandibles while perforating the egg shell and the involvement of different body parts during emergence.
How does your technique compare to conventional high-resolution detectors in practical terms?
Our system demonstrated superior image quality compared to conventional high-resolution detectors at the same X-ray energy. This results on the one hand from the higher efficiency of the detector, but also from a good transfer of high spatial frequencies into the image, while in indirect detectors the optical transfer function of the microscope suppresses high spatial frequencies. These high spatial frequencies are especially important for propagation-based phase contrast and of course for the high resolution.
What other applications do you foresee for your imaging technique?
As an example, biologists at our institute are interested in studying model organisms, especially the embryonic development of the African clawed frog (Xenopus laevis) and the influence of certain genes on the involved processes. The method will enable extended observation times for in vivo studies of cell movement and organ development. Currently, we plan to install the technique at our experimental station at the PETRA III synchrotron of DESY in Hamburg to make dose-efficient imaging available for the user community.
Are there any limitations to the current system, and what improvements might you implement in the future?
The crystals that we are using are 20 cm large, yielding a field of view between 1.5 millimeters and 500 microns, depending on the magnification. In future, we aim to increase the field of view by using even larger crystals. This would also imply using a single-photon-counting detector with an even larger area, which are currently being developed. In addition, we are planning to further increase the mechanical stability of the setup.
Where can readers find more information?
The paper can be found below:
R. Spiecker, P. Pfeiffer, A. Biswal, M. Shcherbinin, M. Spiecker, H. Hessdorfer, M. Hurst, Y. Zharov, V. Bellucci, T. Farago, M. Zuber, A. Herz, A. Cecilia, M. Czyzycki, C. S. Baraldi Dias, D. Novikov, L. Krogmann, E. Hamann, T. van de Kamp, T. Baumbach, “Dose-efficient in vivo X-ray phase contrast imaging at micrometer resolution by Bragg magnifiers,” 10, 12 (2023).
DOI: 10.1364/OPTICA.500978
About Rebecca Spiecker
 Rebecca Spiecker is a Ph.D. student in the group of Professor Tilo Baumbach at the Karlsruhe Institute of Technology, working on X-ray phase contrast imaging. During her physics studies, she worked on light microscopy at the Max-Planck-Institute in Göttingen under Professor Stefan Hell and on phase contrast techniques for electron microscopy in the group of Professor Dagmar Gerthsen at KIT.
Rebecca Spiecker is a Ph.D. student in the group of Professor Tilo Baumbach at the Karlsruhe Institute of Technology, working on X-ray phase contrast imaging. During her physics studies, she worked on light microscopy at the Max-Planck-Institute in Göttingen under Professor Stefan Hell and on phase contrast techniques for electron microscopy in the group of Professor Dagmar Gerthsen at KIT.
Disclaimer: The views expressed here are those of the interviewee and do not necessarily represent the views of AZoM.com Limited (T/A) AZoNetwork, the owner and operator of this website. This disclaimer forms part of the Terms and Conditions of use of this website.