Oct 30 2008
A huge market is developing for small disposable electronic devices, ranging from security tags to point-of-care diagnostics. Many of these devices require a power source, and photovoltaic devices (solar cells) are an attractive option. However, the expense of preparing and processing inorganic semiconductors used in traditional solar cells precludes their use in such applications. Organic photovoltaic devices, meanwhile have great potential in this area; they are relatively easy to prepare and can be processed by simple techniques such as inkjet printing.
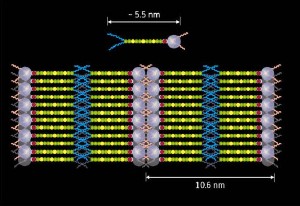 Schematic of the liquid crystal molecule (top) and the formed liquid crystal photovoltaic device (bottom). Purple spheres represent the fullerene and yellow/green chains the oligothiophene, the hydrophobic/hydrophilic tails are represented by blue/red lines respectively.
Schematic of the liquid crystal molecule (top) and the formed liquid crystal photovoltaic device (bottom). Purple spheres represent the fullerene and yellow/green chains the oligothiophene, the hydrophobic/hydrophilic tails are represented by blue/red lines respectively.
Organic photovoltaic devices contain both electron donors, which release an electron when irradiated, and electron acceptors, which complete the circuit necessary to convert light energy into electrical energy. However, mixtures of typical electron donors such as p-conjugated oligomers—short chains of repeated, unsaturated, organic molecules, with alternating double and single bonds—and electron acceptors, such as C60 (buckminsterfullerene), have a tendency to form alternating stacks that results in lower efficiency. A partial solution is to directly attach the electron donor to the electron acceptor by a covalent bond and have both in a single molecule, but it is still important to have control over how the molecules pack together.
Now, a team of Japanese researchers including Takuzo Aida from the University of Tokyo and Masaki Takata from the RIKEN SPring-8 Center in Harima have designed liquid crystals—a phase that flows like a liquid but has short-range order between the molecules—that spontaneously assemble to form a donor-acceptor array*. “It’s important to form separated columns or layers of the donors and acceptors, and to make a large contact area between them,” explains Yohei Yamamoto, another member of the team from the Japan Science and Technology Agency in Tokyo.
The molecules they designed feature a fullerene—the electron acceptor—at one end and a thiophene oligomer—the electron donor—at the other. A hydrophobic, or water-repellent, tail is attached to the donor end and a hydrophilic, or water-loving, tail is attached to the acceptor end. This functionalization ensures that the molecules of the liquid crystal line up to produce ordered layers of donors and acceptors and results in efficient photovoltaic behavior. “The liquid characteristics are useful as well,” notes Yamamoto, “the devices are self-healing as defects in the layer structure can be repaired by a simple heating and cooling process.” The design principles developed in this work should lead to the development of high-efficiency organic photovoltaic devices.
- Li, W.-S., Yamamoto, Y., Fukushima, T., Saeki, A., Seki, S., Tagawa, S., Masunaga, H., Sasaki, S., Takata, M. & Aida, T. Amphiphilic molecular design as a rational strategy for tailoring bicontinuous electron donor and acceptor arrays: photoconductive liquid crystalline oligothiophene–C60 dyads. Journal of the American Chemical Society 130, 8886–8887 (2008).