Reviewed by Lexie CornerApr 5 2024
Iridium oxide catalysts are highly intriguing for green technologies as they work well for oxidizing water. The most thorough examination of their operation to date was conducted by a group of researchers from Osaka University’s SANKEN (The Institute of Scientific and Industrial Research).
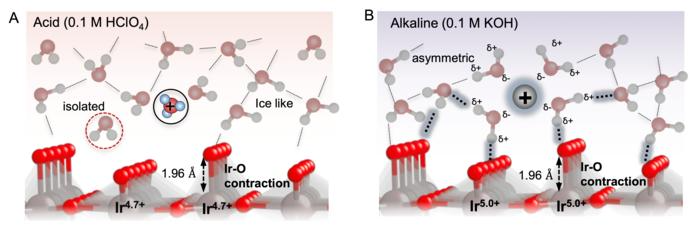 Schematic of electrochemical interface under acidic 0.1 M HClO4 electrolyte (A) and alkaline 0.1 M KOH electrolyte (B) during water oxidation at ~1.5 VRHE. Image Credit: Adapted from the Journal of the American Chemical Society, 2024, DOI: 10.1021/jacs.3c12011
Schematic of electrochemical interface under acidic 0.1 M HClO4 electrolyte (A) and alkaline 0.1 M KOH electrolyte (B) during water oxidation at ~1.5 VRHE. Image Credit: Adapted from the Journal of the American Chemical Society, 2024, DOI: 10.1021/jacs.3c12011
In a study published in the Journal of the American Chemical Society, a team employed spectroscopy to demonstrate how the chemical species involved in the iridium oxide-catalyzed oxygen evolution reaction (OER) interact with the solution around them.
The OER plays a crucial role in several clean energy processes, including turning carbon dioxide into useful liquid fuels and producing green hydrogen through water electrolysis. Both processes will be critical in a future without fossil fuels, so an in-depth understanding of the OER is an essential research focus.
Catalytic processes can be complicated, involving a variety of intermediate species to get from the starting material to the desired product. Operando methods enable spectroscopy to be used to analyze these intermediates throughout the process, offering a window into what is actually occurring.
The researchers used an electrode with an iridium oxide surface to test the oxidation of water molecules in solutions with varying pH levels.
Interaction between the electrode surface and the oxygenated intermediates is key to the efficiency of the OER, so optimizing the catalyst material has generally been the focus. However, the observations to date have left unanswered questions, so we have taken a closer look at the solution side of the interface using operando UV-Vis spectroscopy, X-Ray absorption spectroscopy, and surface-enhanced infrared spectroscopy.
Reshma R. Rao, Study Senior Author and Lecturer, Imperial College London
To produce an effective reaction, the binding of reaction intermediates to the electrode must be just right, allowing the intermediates to interact with the electrode but not so strong that they get stuck and unable to react. The researchers discovered that binding was regulated by long-range interactions between intermediates in the solution, which were pH-dependent.
Water near the electrode in alkaline conditions changed the long-range interactions among the oxygenated species, thus impacting their surface binding. Hence, even if the intermediates are bound more firmly at higher pH values, the reaction is still able to occur because of the interactions made possible by interfacial water, which destabilizes the oxygenated species.
Using operando spectroscopy and complementary techniques to get a direct look at the species involved has allowed us to broaden the understanding of catalyst performance beyond electrode binding. We believe that such insight will be the key to optimizing the kinetics of the OER.
Yu Katayama, Study Senior Author and Associate Professor, The Institute of Scientific and Industrial Research, Osaka University
The results will help improve water oxidation efficiency for the creation of green hydrogen. Moreover, operando spectroscopy could be helpful in understanding the catalysis of several other processes when combined with complementary techniques.
Journal Reference:
Liang, C., et al. (2024) Role of Electrolyte pH on Water Oxidation for Iridium Oxides. Journal of the American Chemical Society. doi:10.1021/jacs.3c12011