Reviewed by Mila PereraSep 20 2022
Surgery is one of the most tried and tested methods of cancer treatment. Despite the availability of radiation therapy, chemotherapy, and novel treatments such as bacteria that target and kill cancer cells, malignancies are frequently cut off from patients’ bodies.
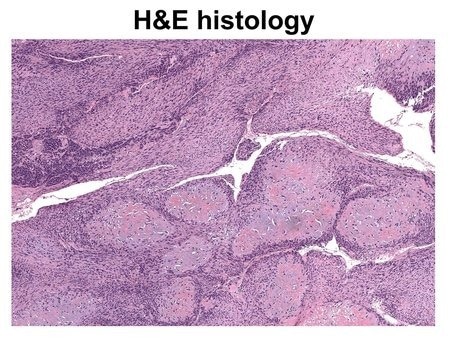 An image of cancerous tissue prepared with the traditional hematoxylin and eosin (H&E) staining method. Image Credit: Caltech.
An image of cancerous tissue prepared with the traditional hematoxylin and eosin (H&E) staining method. Image Credit: Caltech.
The objective is to eliminate all malignant tissue while conserving as much healthy tissue as possible.
As it is difficult to distinguish between cancerous and healthy tissues, surgeons frequently err on the side of caution and excise healthy tissue to ensure that all harmful tissue has been removed.
This removal is particularly problematic when cancer afflicts bones. As bones are harder than other tissues and regenerate slowly, they present unique challenges during surgery.
It's very hard to grow bone, so if you cut out bone, you basically lose it.
Lihong Wang, Bren Professor, Medical Engineering and Electrical Engineering, California Institute of Technology
Investigators at Caltech devised a new diagnostic imaging technology that allows surgeons to make cuts 10 times more accurately, safeguarding around 1,000 times more healthy tissue and providing patients with uncomplicated recoveries.
Conventional methods for determining whether a chunk of bone includes cancer cells take time.
The bone chunk is extracted and transferred to a lab, where the hard calcium matrix is slowly destroyed, leaving just the live cells. After that, the residual material is cut and photographed.
Since the procedure can take anywhere from one to seven days to complete, surgeons cannot depend on it during surgery to assess the quality of the bone around a tumor.
They therefore excise more bone than is required, more than they would in softer tissues that can be biopsied fast.
The novel imaging technology, known as real-time 3-D contour-scan ultraviolet photoacoustic microscopy, or UV-PAM, is intended to replace conventional cancerous bone tissue identification.
As the process occurs within minutes, allowing a surgeon to identify cancerous bone from healthy bone while operating.
UV-PAM, like all other photoacoustic imaging methods invented by Wang, works by vibrating molecules in live tissue using laser light.
These vibrations occur at ultrasonic frequencies and can be used to examine tissues and organs, similar to how ultrasound images a developing fetus.
UV-PAM employs laser light with ultraviolet wavelengths adjusted to cause DNA and RNA molecules to vibrate.
Since cancer cells are structured differently, packed more densely, and contain significantly more DNA than healthy cells, an area of cancerous tissue will absorb more UV light and thus generate a stronger ultrasonic signal than healthy tissue, allowing the surgeon to easily recognize areas of bone that require removal.
We can provide results within 11 minutes, so they know exactly where to cut.
Lihong Wang, Bren Professor, Medical Engineering and Electrical Engineering, California Institute of Technology
This technology provides practitioners with an image of the bone that has been scanned and made to look similar to the images developed by conventional biopsy techniques.
We just present images to the pathologists. They use the same pattern recognition in their own brain to determine what is cancerous and what is healthy. That's their training.
Lihong Wang, Bren Professor, Medical Engineering and Electrical Engineering, California Institute of Technology
This technology is currently demonstrated in a laboratory setting. Wang anticipates applying it to the real world, where it could be used on patients; however, Wang intends to make some improvements.
Wang states, “We would like to give it even finer spatial resolution and greater imaging speed so we could even see some of the details within the cell nucleus more rapidly. Can we go beyond standard pathology? We are working on that.”
The study was published on September 19th, 2022, in the Nature Biomedical Engineering journal.
The co-authors of the study are Rui Cao, a postdoctoral scholar research associate in medical engineering; Scott D. Nelson of UCLA; Samuel Davis, a postdoctoral scholar research associate in medical engineering; Yu Liang of the City of Hope; Yilin Luo, a graduate student in medical engineering; Yide Zhang, postdoctoral scholar research associate in medical engineering; and Brooke Crawford of UCLA.
National Institutes of Health provided funding for the study.
Journal Reference
Cao, R., et al. (2022) Label-free intraoperative histology of bone tissue via deep-learning-assisted ultraviolet photoacoustic microscopy. Nature Biomedical Engineering. doi.org/10.1038/s41551-022-00940-z.