Reviewed by Alex SmithJun 6 2022
Chemical applications that are sustainable must be able to use renewable energy sources, renewable raw materials, and naturally occurring elements. Many techniques, however, have only been plausible with the use of highly-priced precious metals or rare earth metals, whose extraction can have devastating effects on the environment.
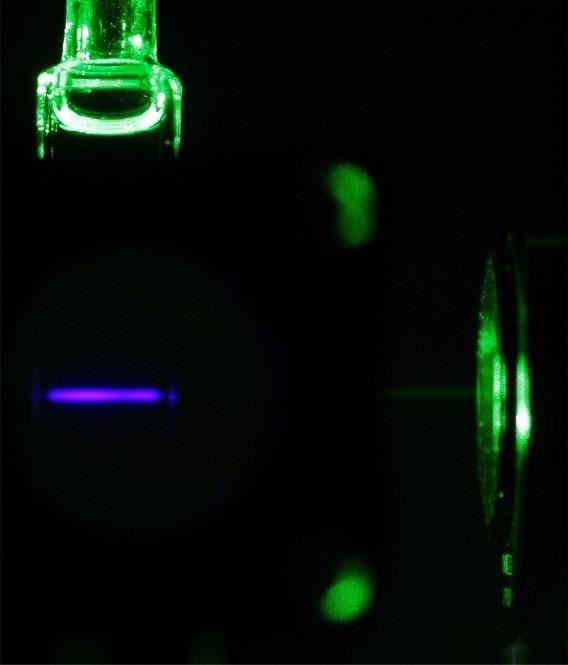 Green-to-blue light upconversion in a solution with a molecular ruby. Image Credit: ©: Yi You
Green-to-blue light upconversion in a solution with a molecular ruby. Image Credit: ©: Yi You
Professor Katja Heinze and Professor Christoph Kerzig of Johannes Gutenberg University Mainz (JGU) as well as Dr. Ute Resch-Genger of the German Bundesanstalt für Materialforschung und -prüfung (BAM) along with other researchers have reached a milestone in the use of chromium, a plentiful base metal that Heinze’s group has been studying for some time.
The latest findings reveal that chromium compounds, also known as molecular rubies, can be used to upconvert photons instead of more expensive precious metals. Photon upconversion (UC) is a process where two lower-energy photons are sequentially absorbed and one higher-energy photon is emitted.
In theory, this higher-energy photon could be used to increase the use of low-energy sunlight in solar cells or photochemical reactions that would otherwise require UV light to activate. Thus, the use of molecular rubies can aid in minimizing the environmental impact of processes like precious metal or rare earth element mining, as well as expanding photochemistry to more sustainable processes.
Chromium Compounds as a Promising Alternative
Precious metals like platinum, gold, iridium, ruthenium, or rare earth metals are used in most photochemical and photophysical applications including dye-sensitized solar cells, phosphorescent organic light-emitting diodes, or light-driven chemical reactions.
Precious metals, on the other hand, are costly due to scarcity, whereas rare earth elements are only mined in a few countries, most notably China. Moreover, their extraction frequently necessitates significant amounts of water, energy, and chemicals. Highly toxic substances like cyanide or mercury are used in some cases, such as gold mining.
On the contrary, the metal chromium, which gets its name from the ancient Greek word for color, is 10,000 times more abundant in the Earth’s crust than platinum and 100,000 times more abundant than iridium, indicating that it is readily available.
Unfortunately, the photophysical properties of abundant metals like chromium or iron are just not good enough to be useful in technological applications, especially when it comes to the lifetimes and energies of their electronically excited states.
Katja Heinze, Professor, Department of Chemistry, Johannes Gutenberg University Mainz
Major progress in this regard took place only in the last few years, with Heinze’s group being one of the major contributors. They were also involved in the study and development of molecular rubies. These are water-soluble molecular compounds with exemplary excited state properties. Molecular rubies have already been used as pressure sensors and molecular optical thermometers.
Direct Observation of the Energy Transfer Processes Thanks to New Large-Scale Laser Device
Another breakthrough has been made by a group of scientists from Mainz and Berlin.
In the process, we observed a novel mechanism and understood the high efficiency of the new chromium compounds in detail.
Christoph Kerzig, Professor, Johannes Gutenberg University Mainz
Using a laser setup installed recently in the Kerzig group, the researchers were able to directly perceive the unusual energy transfer pathway. They were able to identify all intermediates that are essential for the upconversion mechanisms using this so-called laser flash photolysis technique.
Quantitative laser experiments also showed that there are no inherent energy loss channels or side reactions, paving the way for effective applications of this underutilized method of transferring and converting solar energy with chromium compounds.
As a result, researchers may be able to develop new light-driven reactions in the future using the more common metal chromium rather than the rare, more expensive ruthenium and iridium compounds that are currently used.
Together with our partners at BAM in Berlin and other universities we will continue to push on with our efforts to develop a more sustainable photochemistry.
Katja Heinze, Professor, Department of Chemistry, Johannes Gutenberg University Mainz
The team’s findings have been published in Angewandte Chemie, classified as Hot Paper. The research is funded by the German Research Foundation (DFG) and the Chemical Industry Funds. In 2018, the German Research Foundation set up the priority program Light Controlled Reactivity of Metal Complexes (SPP 2102), coordinated by Professor Katja Heinze with the second funding period beginning in 2022.
Journal Reference:
Wang, C., et al. (2022) Efficient Triplet-Triplet Annihilation Upconversion Sensitized by a Chromium(III) Complex via an Underexplored Energy Transfer Mechanism. Angewandte Chemie International Edition. doi.org/10.1002/anie.202202238.