A newly developed multi-frequency method results in a brief measurement time of just a few seconds rather than the traditional 10 minutes.
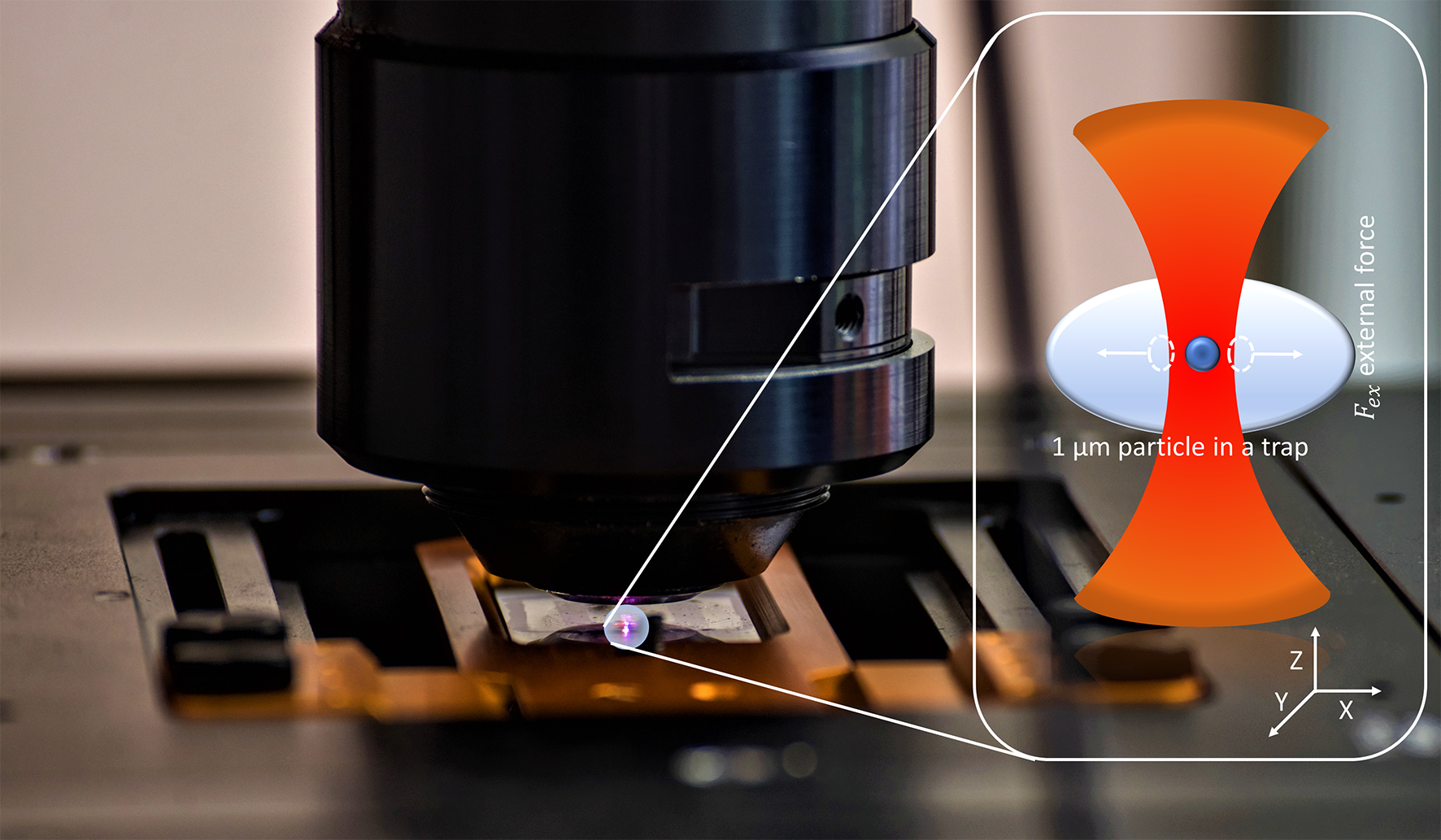 A microparticle held with optical tweezers in the microscope. Inset: Illustration of the held particle (magnified); shown in red is the light of the infrared laser used. Image Credit: © Pascal Runde.
A microparticle held with optical tweezers in the microscope. Inset: Illustration of the held particle (magnified); shown in red is the light of the infrared laser used. Image Credit: © Pascal Runde.
The measurement of biomechanical properties within living cells needs minimally invasive techniques. Specifically, optical tweezers seem to be appealing as a tool.
Optical tweezers make use of the momentum of light to catch and manipulate micro- or nanoscale particles. A research group headed by Professor Dr. Cornelia Denz from the University of Münster has devised a simple technique to carry out the essential calibration of the optical tweezers in the to-be-tested system.
Researchers from the University of Pavia in Italy also contributed to the study, the findings of which have been published in the journal Scientific Reports.
The calibration enables comparable measurements from various samples and with different devices. The purported active-passive calibration is a potential method for calibrating optical tweezers in a viscoelastic medium.
In this method, the deformability of the sample under investigation and the force of the optical tweezers are determined. The team has further enhanced this method to reduce the measurement time. The improved technique enables characterizing dynamic processes of living cells.
These features cannot be analyzed with longer measurements as the cells rearrange themselves during the measurement and alter their properties. The reduction in the measurement time also helps to decrease the threat of damage to the biological samples caused by light-induced heating.
In other words, the fundamental procedure to carry out the calibration works as follows: The micro- or nanometer-sized particles are fitted in a viscoelastic sample positioned on the stage of a microscope.
Quick and accurate nanometer-scale displacements of the specimen stage make the optically trapped particle oscillate. The measurement of the refracted laser light can help record the variations in the position of the sample, thereby enabling its properties like stiffness to be analyzed. Generally, this is performed consecutively at various oscillation frequencies.
The research group headed by Cornelia Denz and Randhir Kumar, a doctoral student in the Münster research group, has currently carried out the measurement at various frequencies at the same time for a broad frequency range.
This multi-frequency technique results in a brief measurement time of just a few seconds rather than the usual 10 minutes. The researcher's utilized solutions of methyl cellulose in water at various concentrations as samples. These exhibit viscoelasticity similar to living cells.
Background
Biomechanical properties like viscosity, stiffness and viscoelasticity of living cells and tissues play a vital role in several vital cellular functions like cell migration, cell division, cell differentiation and tissue patterning. Moreover, such properties of living cells could act as indicators of disease progression. For instance, the onset and growth of cancer are generally accompanied by variations in cell stiffness, viscoelasticity and viscosity.
This study was financially supported by the Cluster of Excellence “Cells in Motion.”
Journal Reference:
Kumar, R., et al. (2021) Multi-frequency passive and active microrheology with optical tweezers. Scientific Reports. doi.org/10.1038/s41598-021-93130-x.