Jan 4 2021
A new technique has been developed by optical physics experts to view inside living cells in more detail by employing present microscopy technology and without the need to add fluorescent dyes or stains.
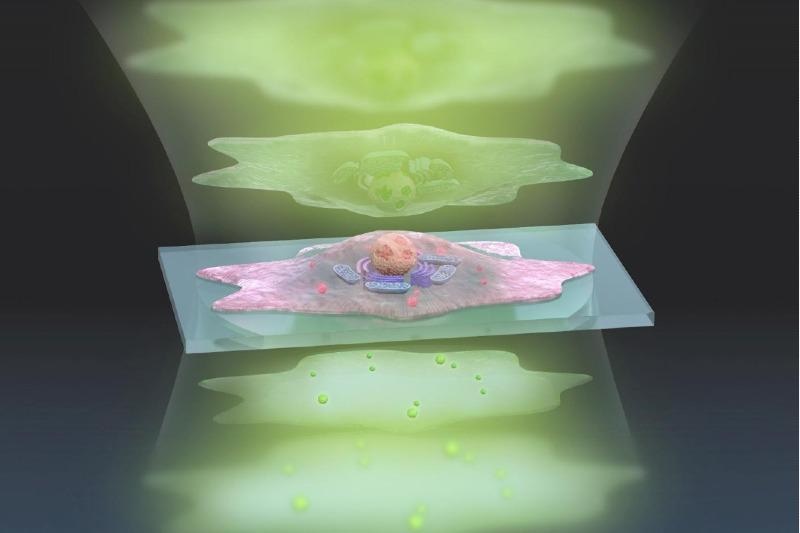 This artistic representation of the ADRIFT-QPI technique shows pulses of sculpted light (green, top) traveling through a cell (center), and exiting (bottom) where changes in the light waves can be analyzed and converted into a more detailed image. Image Credit: s-graphics.co.jp.
This artistic representation of the ADRIFT-QPI technique shows pulses of sculpted light (green, top) traveling through a cell (center), and exiting (bottom) where changes in the light waves can be analyzed and converted into a more detailed image. Image Credit: s-graphics.co.jp.
Considering that individual cells are nearly translucent, microscope cameras should detect extremely delicate differences in the light passing via parts of the cell. Such differences are called the phase of the light. Camera image sensors are restricted by the amount of light phase difference they can detect, which is known as the dynamic range.
To see greater detail using the same image sensor, we must expand the dynamic range so that we can detect smaller phase changes of light.
Takuro Ideguchi, Associate Professor, Institute for Photon Science and Technology, University of Tokyo
A new method was developed by the researchers to take two exposures to quantify small and large changes in the light phase individually and smoothly link them further to make a highly detailed final image. The technique has been dubbed adaptive dynamic range shift quantitative phase imaging (ADRIFT-QPI), and the study results were published recently in the journal Light: Science & Applications.
According to Ideguchi, “Our ADRIFT-QPI method needs no special laser, no special microscope or image sensors; we can use live cells, we don’t need any stains or fluorescence, and there is very little chance of phototoxicity.”
Phototoxicity is the use of light to kill cells and can turn out to be an issue with some other imaging methods, such as fluorescence imaging.
Quantitative phase imaging involves transmitting a pulse of a flat sheet of light toward the cell and then quantifying the phase shift of the light waves as soon as they pass via the cell. Then, computer analysis is used to rebuild an image of the significant structures present within the cell. Ideguchi together with his collaborators has earlier pioneered other techniques to improve quantitative phase microscopy.
Quantitative phase imaging is a robust tool to analyze individual cells since it enables scientists to make elaborate measurements, such as monitoring the growth rate of a cell depending on the shift in light waves.
But the quantitative nature of the method possesses low sensitivity due to the low saturation capacity of the image sensor, and thus it is not viable to monitor nanosized particles in and around cells with a traditional method.
The latest ADRIFT-QPI technique has overcome the dynamic range restriction of quantitative phase imaging. In the ADRIFT-QPI technique, the camera captures two exposures and generates a final image that has seven times greater sensitivity compared to conventional quantitative phase microscopy images.
The initial exposure is generated with traditional quantitative phase imaging—a flat sheet of light is pulsed toward the sample and the shifts in the phase of the light are quantified as soon as it passes via the sample.
An image of the sample is developed by a computer image analysis program based on the initial exposure, where the program then quickly designs a sculpted wavefront of light that reflects that image of the sample. After this, an individual component known as a wavefront shaping device produces this “sculpture of light” with higher intensity light for powerful illumination and pulses it toward the sample for a second exposure.
If the initial exposure generates an image that is considered an ideal representation of the sample, the custom-sculpted light waves of the second exposure would penetrate the sample at various phases, pass via the sample, and then emerge as a flat sheet of light, thereby making the camera to view nothing but a dark image.
This is the interesting thing: We kind of erase the sample’s image. We want to see almost nothing. We cancel out the large structures so that we can see the smaller ones in great detail.
Takuro Ideguchi, Associate Professor, Institute for Photon Science and Technology, University of Tokyo
In practice, the initial exposure is not perfect, and the sculptured light waves appear with delicate phase deviations.
The second exposure shows small light phase differences “washed out” by huge variations in the first exposure. These leftover small light phase differences can be quantified with a higher sensitivity because of the stronger illumination employed in the second exposure.
Further computer analysis rebuilds a final image of the sample with an extended dynamic range from the two measurement results. In proof-of-principle illustrations, the team predicts that the ADRIFT-QPI will generate images with seven times greater sensitivity compared to traditional quantitative phase imaging.
According to Ideguchi, the true advantage of ADRIFT-QPI is its capability to view tiny particles present in the context of the entire living cell without having to use any stains or labels.
For example, small signals from nanoscale particles like viruses or particles moving around inside and outside a cell could be detected, which allows for simultaneous observation of their behavior and the cell’s state.
Takuro Ideguchi, Associate Professor, Institute for Photon Science and Technology, University of Tokyo
Journal Reference:
Toda, K., et al. (2020) Adaptive dynamic range shift (ADRIFT) quantitative phase imaging. Light: Science & Applications. doi.org/10.1038/s41377-020-00435-z.