Jun 11 2020
The main aim of brain research is to understand the source and network of signals as the brain functions. At present, engineers at Carnegie Mellon University have developed a system that can be used for high-density electroencephalography (EEG) imaging of the origin and route of normal and abnormal brain signals.
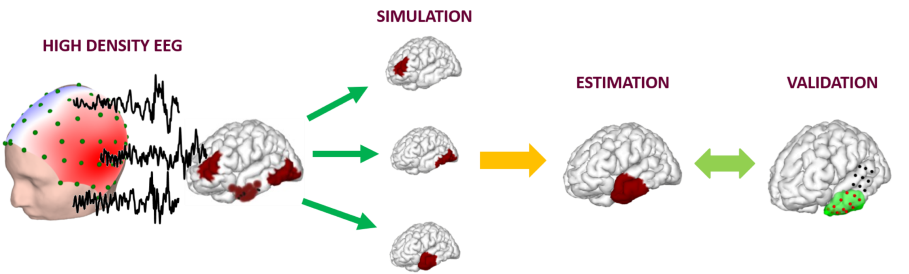 Brain signals are measured with high-density EEG. The signal source is simulated and estimated using artificial intelligence. Results are validated with clinical findings. Image Credit: Carnegie Mellon University College of Engineering.
Brain signals are measured with high-density EEG. The signal source is simulated and estimated using artificial intelligence. Results are validated with clinical findings. Image Credit: Carnegie Mellon University College of Engineering.
Bin He, who is the head of the Department of Biomedical Engineering at Carnegie Mellon University, and his collaborators are focusing on a core initiative of the National Institutes of Health, known as Brain Research through Advancing Innovative Neurotechnologies (BRAIN).
The engineers’ work with high-density EEG has been described in the Nature Communications journal and takes a crucial step forward to achieve one of the central aims of the BRAIN initiative: “produce a revolutionary new dynamic picture of the brain that, for the first time, shows how individual cells and complex neural circuits interact in both time and space.” This means developing a method to observe the functions of the brain.
Electroencephalography (EEG), has been used for decades to track brain signals. Dr. He and his team developed a more powerful, high-density version of EEG that can track brain signals across much larger areas of the brain than previously possible. Artificial intelligence is then used to identify where these stronger signals originate and travel through the brain, with great accuracy.
Shumin Wang, PhD, Director, NIBIB Program in Bio-Electromagnetic Technologies
The high-density EEG system was tested in patients with epilepsy. Such individuals suffer from seizures due to errant pulses of brain activity, called the epileptogenic zone. The seizures of several patients can be controlled with the help of medications.
For people who are medication-resistant, surgical removal of the epileptogenic zone is a clinical option. The capacity of high-density EEG to precisely determine the epileptic source would enable considerably enhanced brain surgeries to efficiently eliminate only the area with a problem while sparing the surrounding brain tissue.
The technique called FAST-IRES, which stands for spatio-temporal iteratively reweighted edge sparsity, was tested on 36 epilepsy patients who underwent pre-operative testing. A unique high-density EEG array was used to obtain FAST-IRES non-invasive brain recordings over a period of several days.
What is very crucial is that FAST-IRES is non-invasive, as against the regular pre-operative testing that needs invasive EEG, which is essential to locate the epileptogenic area but involves higher risks of costs, complications, and infection.
Of the 36 patients, the FAST-IRES technique investigated over 1000 EEG spikes, with 86 seizures. The artificial intelligence piece of the FAST-IRES technique was used to investigate the high-density recordings, which were subsequently compared with the regular invasive EEG pre-operative testing and the surgical results.
The FAST-IRES technique was highly precise in determining the location and degree of the epileptogenic zone in the patients, which was validated with the surgical information.
Our results clearly demonstrated that FAST-IRES could identify the epileptogenic zone with high precision, using non-invasive high-density EEG scalp recordings.
Bin He, Head, Department of Biomedical Engineering, Carnegie Mellon University
At the Mayo clinic, the collaborators on the study are considering the implementation of the system in the future.
The researchers hope that the FAST-IRES method could be employed for the diagnosis and treatment of Parkinson’s, Alzheimer’s, stroke, and even depression.
It is extremely satisfying to see our work make a significant impact in fulfilling one of the central goals of the BRAIN initiative. In addition to treating patients, we look forward to our work helping researchers make significant advances in our understanding of human neuroscience.
Bin He, Head, Department of Biomedical Engineering, Carnegie Mellon University
The study was financially supported by grant EB021027 from the National Institute of Biomedical Imaging and Bioengineering (NIBIB) and grants from the National Institute of Neurological Disorders and Stroke, the National Institute of Mental Health, as well as the National Center for Advancing Translational Sciences.
Journal Reference:
Sohrabpour, A., et al. (2020) Noninvasive Electromagnetic Source Imaging of Spatiotemporally Distributed Epileptogenic Brain Sources. Nature Communications. doi.org/10.1038/s41467-020-15781-0.