Sep 4 2019
An explosion is a complex event in which rapidly changing pressures, temperatures, and chemical concentrations are involved.
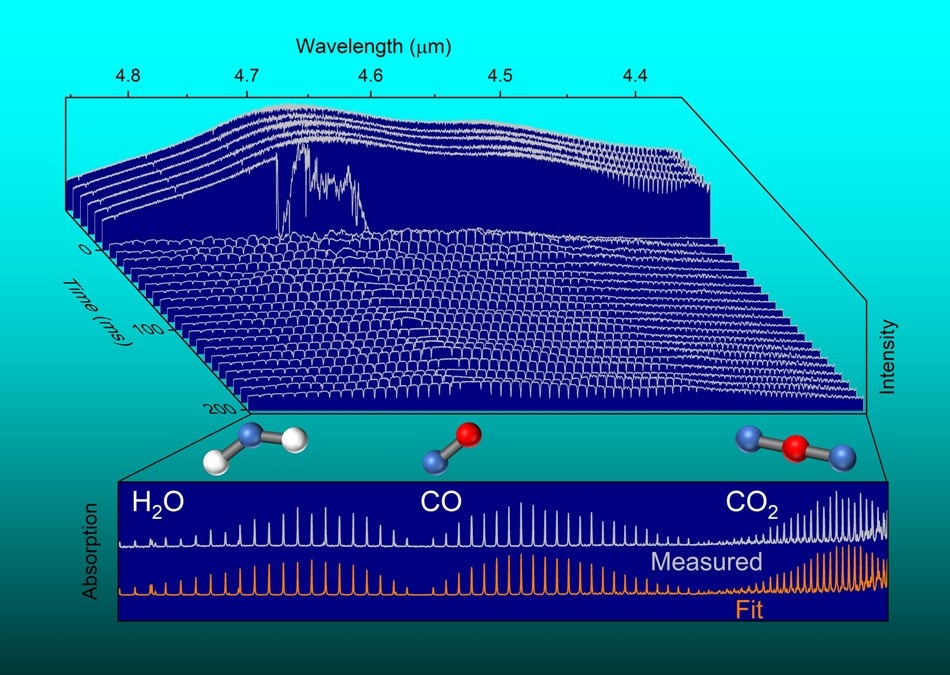 A swept-wavelength external cavity quantum cascade laser measures rapid changes in infrared light absorbed by molecules inside an explosive detonation. (Image credit: Mark C. Phillips)
A swept-wavelength external cavity quantum cascade laser measures rapid changes in infrared light absorbed by molecules inside an explosive detonation. (Image credit: Mark C. Phillips)
In a paper in the Journal of Applied Physics, from AIP Publishing, a unique type of infrared laser, called a swept-wavelength external cavity quantum cascade laser (swept-ECQCL), is used to explore explosions.
This flexible instrument has a widewavelength tuning range that enables the measurement of several chemical substances, even large molecules, in an explosive fireball.
The capabilityof measuring and tracking the dramatic changes during explosions could help researchers understand and even exploit them. Although measurements taken using sturdy pressure or temperature probes placed within an exploding fireball can give physical data, they cannot measure chemical variations that may be produced during the explosion.
Sampling the final products of a detonation is possible but provides information only once the explosion has taken place.
In this research, molecules in the fireball are sensed by tracking the way they interact with light, particularly in the infrared region. These measurements are quick and can be captured a safe distance away. Since fireballs are raging and full of powerfully absorbing substances, lasers are necessary.
Using a novel instrument constructed in their laboratory, the researchers measured explosive events at higher resolutions, at faster speeds, and for longer time periods than earlier possible using infrared laser light.
The swept-ECQCL approach enables new measurements by combining the best features of high-resolution tunable laser spectroscopy with broadband methods such as FTIR.
Mark Phillips, Study Co-Author, University of Arizona
The researchers studied four types of high-energy explosives, all placed in a specifically built chamber to contain the fireball. A laser beam from the swept-ECQCL was passed through this chamber while quickly varying the wavelength of the laser light. The laser light transmitted through the fireball was captured throughout each explosion to measure variations in the way infrared light was trapped by molecules in the fireball.
The explosion creates substances like carbon monoxide, carbon dioxide, nitrous oxide, and water vapor. These can all be spotted by the characteristic way each substance absorbs infrared light.
Comprehensive analysis of the results offered the team with information regarding concentrations and temperature of these substances during the explosive event. They were also able to compute absorption and emission of infrared light from minute solid particles (soot) produced by the explosion.
The swept-ECQCL measurements offer a new approach for exploring explosive detonations that could have other uses. Going forward, the researchers hope to spread the measurements to more wavelengths, higher resolutions, and faster scan rates.