Feb 13 2019
Laser physicists have captured the reaction of C60 carbon molecules to very short pulses of intense infrared light as snapshots.
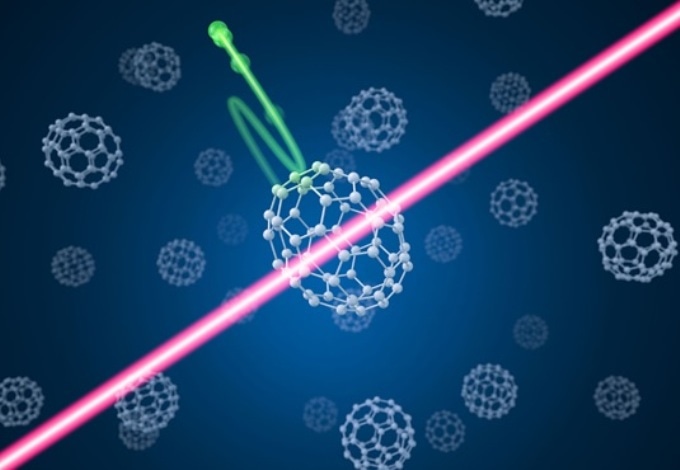 An infrared laser pulse hits a carbon macromolecule. This induces a structural transformation of the molecule and releases an electron into the environment. The laser-induced diffraction of the electron is used to image the transformation. (Image credit: Alexander Gelin)
An infrared laser pulse hits a carbon macromolecule. This induces a structural transformation of the molecule and releases an electron into the environment. The laser-induced diffraction of the electron is used to image the transformation. (Image credit: Alexander Gelin)
C60 is a very well-analyzed carbon molecule that is made of 60 carbon atoms, with a structure similar to that of a soccer ball. The macromolecule is also referred to as buckminsterfullerene, or buckyball, a name given in the remembrance of Richard Buckminster Fuller, an architect who designed buildings with similar shapes.
Currently, laser physicists have irradiated buckyballs using infrared femtosecond laser pulses (one femtosecond is one-millionth of one-billionth of 1 second). The shape of the macromolecule changed from round to elongated under the impact of the intense light.
The physicists could view this structural transformation with the help of the following trick: The infrared pulse, at its greatest strength, activated the release of an electron from the molecule. The oscillations in the light’s electromagnetic field make the electron to be first accelerated away from and subsequently drawn back toward the molecule, all within the duration of a few femtoseconds. Eventually, the electron was released off the molecule and left it completely. From the images of these diffracted electrons, it was possible to reconstruct the molecule’s deformed structure.
The Nobel Prize in Chemistry for the year 1996 was awarded for the discovery of fullerenes, which are biocompatible, stable, and show exceptional chemical, physical, and electronic properties.
A deeper understanding of the interaction of fullerenes with ultrashort, intense light may result in new applications in ultrafast, light-controlled electronics, which could operate at speeds many orders of magnitude faster than conventional electronics.
Dr Matthias Kling, Professor, Ludwig Maximilian University of Munich.