Jan 3 2019
A group of engineers and neuroscientists has created a small, implantable device that could possibly eliminate the need for electronic stimulators or medication in patients suffering from bladder problems.
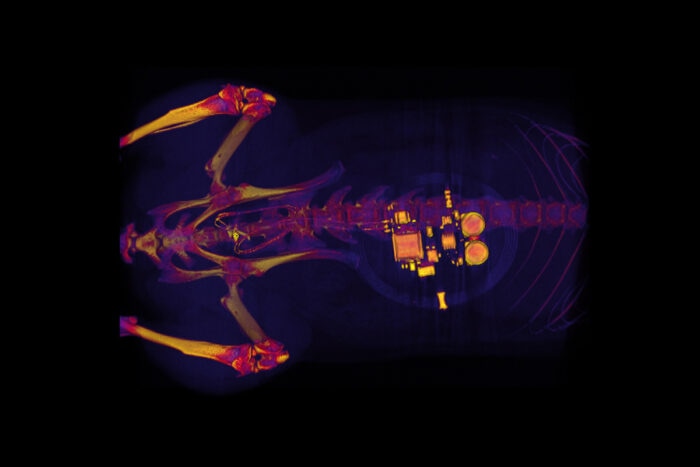 This CT scan of a rat shows a small device implanted around the bladder. The device—developed by scientists at Washington University School of Medicine in St. Louis, the University of Illinois, and Northwestern University—uses light signals from tiny LEDs to activate nerve cells in the bladder and control problems such as incontinence and overactive bladder. (Image credit: GEREAU LAB, Washington University)
This CT scan of a rat shows a small device implanted around the bladder. The device—developed by scientists at Washington University School of Medicine in St. Louis, the University of Illinois, and Northwestern University—uses light signals from tiny LEDs to activate nerve cells in the bladder and control problems such as incontinence and overactive bladder. (Image credit: GEREAU LAB, Washington University)
The soft, implantable device, developed by the research team from Washington University School of Medicine in St. Louis, the Feinberg School of Medicine at Northwestern University in Chicago, and the University of Illinois at Urbana-Champaign, is capable of detecting overactivity in the bladder and subsequently using light from very small, bio-integrated LEDs to reduce the frequent need to urinate.
Currently, the novel device works only in laboratory rats, but in the future, it may help people who frequently have the urge to urinate or suffer from incontinence. The latest strategy has been described in an article published in the journal Nature on January 2nd, 2019.
Some common and distressing problems are pain, burning sensation, overactive bladder, and a frequent urge to urinate. For almost three decades, many people suffering from severe bladder problems have been commonly treated with stimulators that deliver an electric current to the nerve controlling the bladder. While such implants improve overactive bladder and incontinence, they are also known to impact normal nerve signaling to other organs.
“There definitely is benefit to that sort of nerve stimulation,” stated Robert W. Gereau IV, PhD, one of the study’s senior investigators and the Dr Seymour and Rose T. Brown Professor of Anesthesiology at Washington University School of Medicine. “But there also are some off-target side effects that result from a lack of specificity with those older devices.”
In order to prevent such side effects, Gereau and his coworkers created the novel device.
The researchers typically implant a stretchy and soft belt-like device around the bladder during a minor surgical procedure. This belt expands and contracts as the bladder fills and empties. The team also injects proteins known as opsins into the bladders of animals. A virus bound to the bladder nerve cells carries the opsins and makes those cells sensitive to light signals. This mechanism enables the investigators to apply optogenetics to stimulate those nerve cells. In optogenetics, light is used to regulate cell behavior in living tissues.
Through Bluetooth communication to signal an external hand-held device, the researchers can read data in real time, and with the help of a simple algorithm, they can identify when the bladder is full, when the bladder is emptying too frequently, and when the animal has emptied its bladder.
“When the bladder is emptying too often, the external device sends a signal that activates micro-LEDs on the bladder band device, and the lights then shine on sensory neurons in the bladder. This reduces the activity of the sensory neurons and restores normal bladder function,” said Gereau.
According to the researchers, a similar approach may also work in people. For people, devices might be larger than the ones being applied in rats, and could be implanted without any need for surgery, utilizing catheters to place them into the bladder via the urethra.
We’re excited about these results. This example brings together the key elements of an autonomous, implantable system that can operate in synchrony with the body to improve health: a precision biophysical sensor of organ activity; a noninvasive means to modulate that activity; a soft, battery-free module for wireless communication and control; and data analytics algorithms for closed-loop operation.
John A. Rogers, PhD, Professor, Department of Materials Science and Engineering, Northwestern University.
Rogers is the other senior investigator of the study.
The term closed-loop operation actually means that the device provides the therapy only upon detecting a problem. Once the behavior is normalized, the micro-LEDs are switched off and the treatment can be discontinued.
Rogers and Gereau are looking forward to test analogous devices in larger animals. The team also believes that the new approach could be applied to other parts of the body—for instance, treating chronic pain or utilizing light to activate pancreatic cells to secrete insulin. However, one barrier involves the viruses used to obtain light-sensitive proteins to bind to the organs’ cells.
We don’t yet know whether we can achieve stable expression of the opsins using the viral approach and, more importantly, whether this will be safe over the long term. That issue needs to be tested in preclinical models and early clinical trials to make sure the strategy is completely safe.
Robert W. Gereau IV, PhD, Dr Seymour and Rose T. Brown Professor of Anesthesiology, Washington University School of Medicine.
The study was supported by an NIH Director’s Transformative Research Award, an NIH SPARC Award from the National Institute of Biomedical Imaging and Bioengineering, the National Institute of Neurological Disorders and Stroke, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of General Medical Sciences, and the National Institute on Drug Abuse, of the National Institutes of Health (NIH), grant numbers TR01 NS081707, U18 ED021793, R01 NS42595, F32 DK115122, T32 DA007261, T32 DK108742, T32 GM108539, DK082315, and K08 DK094964, and the Dominantly Inherited Alzheimer’s Network, DIAN UF1AG032438.
Additional funding came from the McDonnell Center for Cellular and Molecular Neurobiology, the Urology Care Foundation Research Scholars Program and the Kailash Kedia Research Scholar Endowment, the National Science Foundation, the Ryan Fellowship, and the Northwestern University Institute for Nanotechnology.