Jul 11 2017
A novel design strategy for efficient light-emitting molecules with applications in next-generation lighting and displays has been established as a result of the improved investigation of a molecule that was initially synthesized with the aim of developing a unique light-absorbing pigment.
 (CREDIT William J. Potscavage, Jr.)
(CREDIT William J. Potscavage, Jr.)
Researchers at Kyushu University's Center for Organic Photonics and Electronics Research (OPERA) have demonstrated that a molecule will be able to attain a high efficiency in organic light-emitting diodes (OLEDs) by slightly changing its chemical structure before and after emission.
Besides developing vibrant colors, OLEDs can also be fabricated into almost everything ranging from small pixels to huge and flexible panels, enabling them to be majorly attractive for lighting and displays.
In an OLED, electrical charges that are injected into thin films of organic molecules gather together to produce packets of energy, known as excitons, which are capable of producing light emission.
The aim here is to transform all of the excitons to light, however three-fourths of the developed are triplets, which do not generate light in standard materials, whereas the remaining one-fourth are singlets, which are capable of emitting via a process known as fluorescence.
The addition of a rare metal, such as platinum or iridium, in a molecule is capable of bringing about rapid emission from the triplets via phosphorescence, which is presently considered to be the leading technology for OLEDs that are highly efficient.
An optional mechanism refers to the use of heat in the environment in order to provide triplets an energetic boost, which is adequate for transforming them into light-emitting singlets.
This process, called thermally activated delayed fluorescence (TADF), effortlessly takes place at room temperature in suitably designed molecules and comprises of the added benefit of preventing the cost and reducing molecular design freedom linked with rare metals.
However, most TADF molecules continue to depend on the same fundamental design approach.
Many new TADF molecules are being reported each month, but we keep seeing the same underlying design with electron-donating groups connected to electron-accepting groups. Finding fundamentally different molecular designs that also exhibit efficient TADF is a key to unlocking new properties, and in this case, we found one by looking at the past with a new perspective.
Masashi Mamada, Lead Researcher of the study
Presently, combinations of donating and accepting units are chiefly used as they offer a fairly simple way to push around the electrons in a molecule and attain the conditions required for TADF.
The quest to find unique or perfect emitters still needs new strategies even though a wide range of combinations are possible and the method is effective.
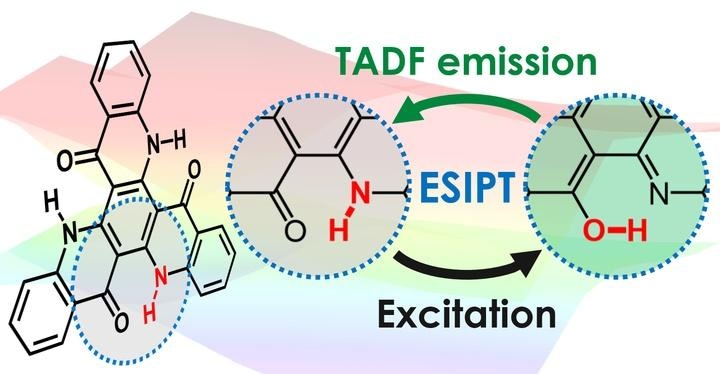
The Researchers have now explored the mechanism involving the reversible transfer of a hydrogen atom - technically, only its positive nucleus – from a single atom in the emitting molecule to in the same molecule in order to produce an arrangement conducive to TADF.
This transfer spontaneously takes place when the molecule is excited with electrical or optical energy and is called excited-state intramolecular proton transfer (ESIPT).
This ESIPT process is considered to be extremely vital in the investigated molecules that quantum chemical calculations by the Researchers point out that TADF cannot be performed before transfer of the hydrogen.
The hydrogen rapidly transfers to a different atom in the molecule after excitation, and this leads to a molecular structure capable of TADF.
After the molecule emits light, the hydrogen transfers back to its initial atom, and the molecule is now ready to repeat the process.
Despite the fact that TADF from an ESIPT molecule has been earlier reported, this indeed is the very first demonstration of greatly efficient TADF observed outside and inside a device.
This immensely different design strategy makes room for obtaining TADF with a range of new chemical structures that would not have been taken into account based on earlier strategies.
Interestingly, the Researchers used the molecule that turned out to be almost a disappointment when initially synthesized almost 20 years ago by Chemists with the hope of developing a new pigment just to discover that the molecule is colorless.
Organic molecules never cease to amaze me. Many paths with different advantages and disadvantages exist for achieving the same goal, and we have still only scratched the surface of what is possible.
Professor Chihaya Adachi, Director of OPERA
The benefits of this design strategy are only now beginning to be analyzed, however one specifically promising area is linked to stability.
Molecules that are similar to the investigated one are considered to be greatly resistant to degradation, hence Researchers believe that such molecules could help enhance the lifetime of OLEDs.
Tests are being performed to see if this is the case.
The constantly developing options for OLED emitters undoubtedly bode well for their future, while only time will be able to reveal the extent to which this particular strategy can be used.