Apr 18 2017
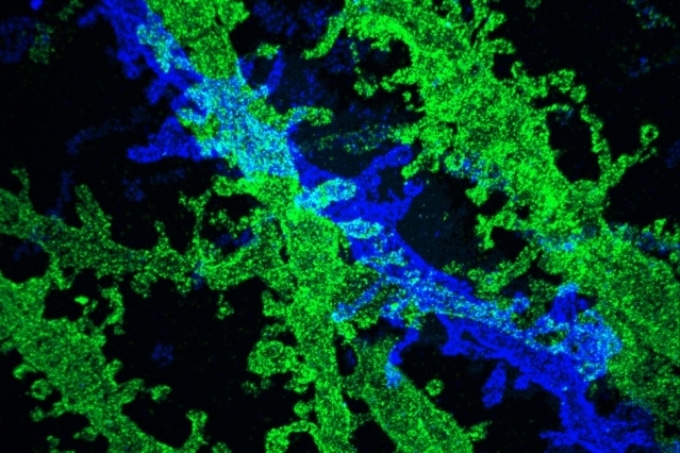 By expanding brain tissue twice, researchers were able to obtain high-resolution images of neurons in the hippocampus. (Image courtesy of the researchers.)
By expanding brain tissue twice, researchers were able to obtain high-resolution images of neurons in the hippocampus. (Image courtesy of the researchers.)
Scientists at MIT have discovered a process for making exceptionally high-resolution images of tissue samples, but at a cost that is considerably less when compared to other methods that provide similar resolution.
The new method involves expanding tissue before imaging the sample using a traditional light microscope. Before two years, the MIT researchers demonstrated that tissue volumes can be expanded 100 times, leading to an image resolution of nearly 60 nm.
At present, the researchers have demonstrated that the resolution can be enhanced to nearly 25 nm by expanding the tissue once more before being imaged.
Such a resolution enables the researchers to observe, for instance, the proteins clustering together under complex patterns near brain synapses, thus assisting communication among neurons.
According to Ed Boyden—an associate professor of biological engineering and brain and cognitive sciences from MIT—it can also enable mapping neural circuits.
We want to be able to trace the wiring of complete brain circuits. If you could reconstruct a complete brain circuit, maybe you could make a computational model of how it generates complex phenomena like decisions and emotions. Since you can map out the biomolecules that generate electrical pulses within cells and that exchange chemicals between cells, you could potentially model the dynamics of the brain.
Ed Boyden, Associate Professor, MIT
The technique can be also applied for imaging various phenomena such as interactions among immune cells and cancer cells, for mapping cell types of the body, and for detecting pathogens without the need for high-cost equipment.
The paper was published in the journal Nature Methods on 17 April, and the first author of the paper is former MIT postdoc, Jae-Byum Chang.
Double expansion
The tissue samples were expanded by embedding them in an evenly generated, dense gel formed of polyacrylate—a highly absorbent material used in diapers. Before forming the gel, the cell proteins to be imaged are labeled by means of antibodies bound to particular targets.
The antibodies carry “barcodes” formed of DNA, which is in turn bound to cross-linking molecules attached to polymers that form the expandable gel.
Then, the proteins that generally hold the tissue together are broken down, thus enabling the expansion of the DNA barcodes away from one another once the gel expands.
Then, fluorescent probes bound with the DNA barcodes can be used to label the enlarged samples. Next, the enlarged samples are imaged by means of commercially obtainable confocal microscopes with a resolution that is normally restricted to just few hundred nanometers.
With this technique, the scientists could earlier achieve a resolution of nearly 60 nm. Yet, “individual biomolecules are much smaller than that, say 5 nanometers or even smaller,” stated Boyden.
The original versions of expansion microscopy were useful for many scientific questions but couldn’t equal the performance of the highest-resolution imaging methods such as electron microscopy.
Ed Boyden, Associate Professor, MIT
Working on an original expansion microscopy research, the research team discovered that the tissue samples can be expanded over 100 times in volume by decreasing the number of cross-linking molecules holding the polymer in an orderly fashion; but this rendered the tissue unstable.
“If you reduce the cross-linker density, the polymers no longer retain their organization during the expansion process,” stated Boyden, a member of MIT’s Media Lab and McGovern Institute for Brain Research. “You lose the information.”
Alternatively, the research team, in a very recent research, altered their method such that a new gel that expands the tissue another time after the first tissue expansion was developed—a technique known as “iterative expansion.”
Mapping circuits
The research team employed iterative expansion to image the tissue samples at a resolution of nearly 25 nm, similar to that achieved by high-resolution methods like stochastic optical reconstruction microscopy (STORM).
Still, according to Boyden, expansion microscopy is considerably inexpensive and simple to carry out as it does not necessitate customized chemicals or equipment. Moreover, the technique is way faster and hence compatible with 3D imaging on a large scale.
In terms of resolution, expansion microscopy is not efficient like transmission electron microscopy (nearly 1 nm) or scanning electron microscopy (nearly 5 nm). Yet, electron microscopes are quite costly and are not easily available. Also, labeling of particular proteins may be challenging with electron microscopes.
In the paper published in Nature Methods, the MIT researchers employed iterative expansion to image synapses, that is, the connections among neurons that enable communication among the neurons.
In the original expansion microscopy research carried out by the researchers, they were successful in imaging scaffolding proteins that play a vital role in organizing the various other proteins that occur in synapses.
The new, improved resolution enabled the research team to observe finer structures, for example, the location of neurotransmitter receptors on the “postsynaptic” cells’ surfaces on the receiving side of the synapse.
“My hope is that we can, in the coming years, really start to map out the organization of these scaffolding and signaling proteins at the synapse,” stated Boyden.
Boyden considers that this can be achieved by combining expansion microscopy with an innovative technique known as temporal multiplexing. At present, only very few colored probes can be used for imaging disparate molecules in tissues.
Temporal multiplexing enables labeling a molecule using a fluorescent probe, taking an image, and finally washing the probe away. This process can be repeated multiple times, where the same colors are used each time to label disparate molecules.
By combining iterative expansion with temporal multiplexing, we could in principle have essentially infinite-color, nanoscale-resolution imaging over large 3-D volumes. Things are getting really exciting now that these different technologies may soon connect with each other.
Ed Boyden, Associate Professor, MIT
The research team believes that a third expansion can be performed, which can generally ensure a resolution of nearly 5 nm. Yet, at present, the resolution is restricted by the size of the antibodies that are used for labeling the molecules in the cell, which is about 10-20 nm long.
Therefore, to achieve an even better resolution, either smaller tags must be created or the proteins must be expanded away from each other first and the antibodies must be delivered after the expansion.
The National Institutes of Health Director’s Pioneer Award, the New York Stem Cell Foundation Robertson Award, the HHMI-Simons Faculty Scholars Award, and the Open Philanthropy Project funded this research.