Jun 14 2016
Aggressive neuroendocrine cancer is something of a dark horse—a rare, elusive and persevering force linked to discouraging long-term survival rates. Researchers at the 2016 Annual Meeting of the Society of Nuclear Medicine and Molecular Imaging (SNMMI) are presenting a molecular imaging technique that allows oncologists to set patients’ radiotherapy doses right at that critical limit of delivering the most powerful kill to neuroendocrine tumors (NETs) while protecting vulnerable vital organs.
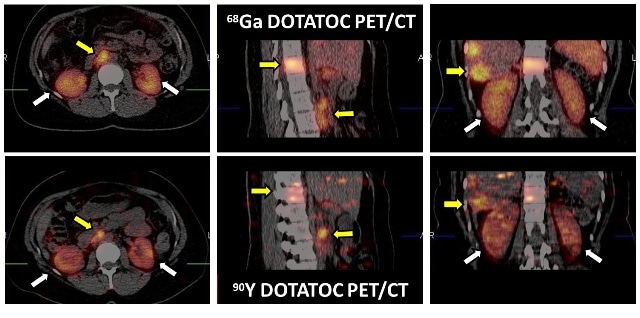 Comparison of Ga-68-DOTATOC PET/CT with Y-90 DOTATOC PET/CT. Although the positron associated with Y-90 is rarely emitted, there is still sufficient signal to acquire a quantitative PET/CT image of Y-90 DOTATOC after a therapeutic administration. The white arrows indicate kidneys and the yellow arrows tumor.(Credits: University of Iowa)
Comparison of Ga-68-DOTATOC PET/CT with Y-90 DOTATOC PET/CT. Although the positron associated with Y-90 is rarely emitted, there is still sufficient signal to acquire a quantitative PET/CT image of Y-90 DOTATOC after a therapeutic administration. The white arrows indicate kidneys and the yellow arrows tumor.(Credits: University of Iowa)
The delicate balance of administering the maximum safe dose is called personalized dosimetry, and it can vary widely between patients. This poses a problem for clinicians. A number of radiotherapies that marry a small but potent amount of radioactive material and a targeted molecular compound have been gaining traction as progressive treatments for malignant NETs, which can develop wherever nerve cells and hormone-producing endocrine cells are present (e.g., gastrointestinal tract, pancreas, lungs, thyroid). Scientists are taking preemptive action by using already available molecular imaging systems to determine the optimal dose of one such peptide-receptor radionuclide therapy known as yttrium-90 DOTA0-Tyr3-octreotide (Y-90 DOTATOC).
“DOTATOC is a peptide that binds with somatostatin receptors that are often highly expressed in neuroendocrine cancers,” said Mark T. Madsen, PhD, University of Iowa in Ioa City, Iowa. “With molecular imaging, we are able to see whether the DOTATOC imaging agent is taken up by the tumor. If it is, we know that the Y-90 DOTATOC radiotherapy will also reach the tumor and be able to kill tumor cells.”
The objective here is to set the bar for the highest possible therapeutic radiation dose to NETs that does not exceed toxic radiation levels to the kidneys, which take on the brunt of the residual drug that does not bind to its targets. Results of this research showed that the use of positron emission tomography (PET) and single photon emission computed tomography (SPECT), which image physiological functions of the body such as peptide receptor activity, led to dramatically altered dosimetry in all participating patients.
For this study, researchers worked with 12 patients with malignant NETs to find an adaptive approach to personalized dosimetry during three cycles of radiotherapy. All adult patients underwent 4.44 GBq of Y-90 DOTATOC and pediatric patients received 1.85 GBq per m2 during the first course of treatment. Patients were also given an infusion of amino acids as a protective measure against kidney toxicity. The researchers evaluated blood and renal dosimetry after the first and second courses of radiotherapy to discern the best possible dose of Y-90 DOTATOC for successive courses. Dosimetry was performed by imaging patients with quantitative PET/CT about five hours after administration of Y-90 DOTATOC, followed by quantitative SPECT/CT imaging at 24, 48 and 72-hour intervals after injection. Y-90 burden and kidney mass were determined from reconstructed PET and CT images and verified using phantoms—inanimate objects that act just like patients’ vital organs during imaging and therapy.
The researchers were able to complete 20 dosimetry evaluations for the 12 subjects involved in the study. Y-90 activity accumulation in the kidneys ranged from 1.4 to 3.6 percent. The kidney dose ranged from 0.6 to 2.7 mGy per MBq, and the blood dose ranged from 0.04 to 0.24 mGy per MBq. This led to marked changes in subsequent cycles of treatment. The dose of radiotherapy was not increased for two injections in children, and one patient’s treatment ended after only one course of treatment. For the rest of the subjects, the dose of radiotherapy was increased in eight courses and decreased in three courses. Over all, the prescribed dose of radiotherapy was altered by more than 15 percent in a total of 11 courses of radiotherapy.
“Our approach combines the advantages of quantitative Y-90 PET and SPECT imaging to gather all the information required to accurately estimate kidney dose,” said Madsen. “We expect better outcomes in radionuclide therapy treatment with fewer complications because we will be able to adjust patient dose either up or down as needed.”
While this method of personalized dosimetry was used to determine the maximum dose that is safe for kidneys, this imaging technique could be equally used to determine the most effective dose to combat NETs. Further studies should elucidate how best to apply this technique to improve the standard of neuroendocrine cancer care.