Mar 28 2016
Electric pacemakers are used by people worldwide to help control abnormal heartbeats. The devices have helped scientists learn more about the heart’s physiology and disorders, says Chao Zhou, but they have their limits. Pacemakers must be surgically implanted. They can cause unwanted contractions in other areas of the chest, alter pH levels and cause tissue damage.
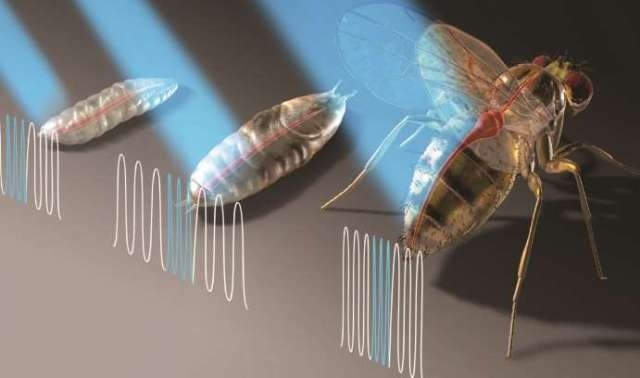 Chao Zhou and his collaborators used optogenetics to noninvasively monitor and analyze the structure and function of the fruit fly heart at the larval, pupal and adult stages.
Chao Zhou and his collaborators used optogenetics to noninvasively monitor and analyze the structure and function of the fruit fly heart at the larval, pupal and adult stages.
Zhou, an assistant professor of electrical and computer engineering, and his colleagues have taken the first step toward developing a laser pacemaker that could one day stimulate the human heart noninvasively with light signals.
The researchers have built a microscope that paces the heart of the common fruit fly without touching it, while controlling its function, monitoring its performance and taking high-resolution images of it at the microscale.
The system uses two relatively new optical technologies—optical coherence tomography (OCT) and optogenetics—to generate pulsed blue light signals that pace the fruit fly’s heart during the three stages of its life: larva, pupa and adult.
The group reported its results recently in Science Advances, a Science magazine journal. Their article, “Optogenetic pacing in Drosophila melanogaster,” was written by Aneesh Alex, a former postdoctoral research scientist at Lehigh, and coauthored by Zhou and by Airong Li and Rudolph E. Tanzi of Harvard Medical School’s Department of Neurology. The project has been funded by the National Institutes of Health (NIH).
Optogenetics uses light to control and study the activities of living cells that have been genetically modified with a light-sensitive protein. OCT combines light waves (usually near infrared) with interferometry to capture microscale images from deep within biological tissue and other media that scatter optical signals.
As a postdoctoral researcher five years ago, Zhou worked with Harvard Medical School researchers to study fruit flies using OCT. “I came up with a new idea,” he says, “which was to use optogenetics to stimulate heart pacing and OCT to monitor heart function.
“We found we could shine a light to control the heart rhythm and use OCT to confirm that the heart was beating and to see how it was beating in real time.”
OCT, says Zhou, is similar to ultrasound, which sends sound waves into tissue and measures the reflections.
“OCT uses near infrared light, which has a shorter wavelength than sound waves and gives us much finer microscale resolution. As the light is reflected from different depths of tissue, the delay in the reflecting of the light signal tells you how deep the signal has penetrated into tissue. From this, we generate a cross-section of a sample from beneath the surface of the tissue.”
The fruit fly, known scientifically as Drosophila melanogaster, offers advantages for optical study, says Zhou. Biologists have obtained the complete genome sequence for the fly and developed ways to modify the genome. And despite differences in scale and complexity between humans and fruit flies, Zhou says, the genomes of the two are similar.
“For human beings and fruit flies,” says Zhou, “70 to 80 percent of the genome is the same. You can easily test a human gene type in the fruit fly and then in a mouse.”
The heart of a typical adult fly lies about 200 microns below the fly’s outer tissue surface. It varies in size between 10 and 20 microns when contracting and about 100 microns when dilating. It beats 300 to 400 times per minute.
“It is possible to observe the heartbeat of an intact fly only with near-infrared OCT technology,” says Zhou. “Because the fruit fly heart is so small, we need an imaging technique with a high resolution that works quickly. Otherwise, the image we get is blurry.”
Zhou and his group bred a genetically altered strain of fruit flies by inserting a light-sensitive protein (taken from algae) into their cardiac cells. They anesthetized a fly, taped its wings to a glass slide and shined a low-power, 16-milliwatt blue laser at its heart. When they altered the frequency of the laser, the fly’s heart rate changed accordingly. When the laser pulsed 10 times per second, for example, the heart rate accelerated to 10 beats per second.
To monitor the fruit fly’s response to the laser, Zhou and his group developed an optical coherence microscopy (OCM) system that enables nondestructive microscale imaging in real time. They were able to monitor and analyze the structure and function of the fly heart at the larval, pupal and adult stages. OCM, they wrote in Science Advances, allowed the group to “quantitatively determine cardiac physiological parameters, such as refractory period [recovery time] and contraction time, of the Drosophila heart at different stages of its life cycle.”
“One of the key advantages of our system is that it is completely noninvasive,” says Zhou. “We can do experiments again and again on the same specimen and see how the same fly heart grows and develops.”
During their three-year study, Zhou and his collaborators have observed that the fruit fly’s heart slows down in the early pupal stage, stops beating for about a day, and resumes beating at its normal heart rate of 300-400 beats per minute in the late pupal and adult stages.
“We were the first group to observe this dynamic change in the fly heart rate,” says Zhou. “It is difficult to detect this without the noninvasive imaging tools we have.”
Zhou has a second NIH grant to explore different pacing strategies using OCM. He says it will take many years before optical pacemakers can be used in humans.
“Our initial goal was not to develop optogenetic pacing for humans,” he says. “Instead, we wanted to do this in small animals to verify and refine the effectiveness of using near-infrared light signals to stimulate and image heart muscle cells. Also, we wanted to find out how different genes affect heart development. To learn this, we first needed to develop a good research tool.
“With bigger animals come challenges. How do you insert light-sensitive proteins? How do you do near-infrared stimulation? An optical fiber or LED would need to be implanted, which is invasive.”
Improving the ability to do noninvasive pacing with optical signals, however, can also help advance the development of novel medical treatments, says Zhou.
“For example, if a person is born with a congenital defect in his heart, can we shine a light on his heart early in his developmental stages, to help correct the defect? Can we use therapeutic tools to correct human heart diseases?
“We don’t know the answer to these questions yet, but the technology now exists to make it possible to think about them.”