Dec 15 2015
A new material that is both highly transparent and electrically conductive could make large screen displays, smart windows and even touch screens and solar cells more affordable and efficient, according to materials scientists and engineers at Penn State who have discovered just such a material.
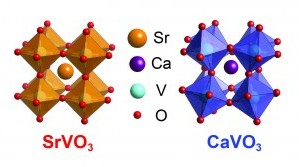 A figure showing the crystal structure of strontium vanadate(orange) and calcium vanadate (blue). The red dots are oxygen atoms arranged in 8 octohedra surrounding a single strontium or calcium atom. Vanadium atoms can be seen inside each octahedron.
A figure showing the crystal structure of strontium vanadate(orange) and calcium vanadate (blue). The red dots are oxygen atoms arranged in 8 octohedra surrounding a single strontium or calcium atom. Vanadium atoms can be seen inside each octahedron.
Indium tin oxide (ITO), the transparent conductor that is now used for more than 90 percent of the display market, has been the dominant material for the past 60 years. But in the last decade, the price of indium has increased dramatically. Displays and touchscreen modules have become a main cost driver in mobile devices, such as smartphones and tablets, making up close to 40 percent of the cost. While memory chips and processors get cheaper, following Moore’s Law, smartphone and tablet displays get more expensive from generation to generation. Manufacturers have searched for a possible ITO replacement, but until now, nothing has matched ITO’s combination of optical transparency, electrical conductivity and ease of fabrication.
In a paper appearing today(Dec 15) online in Nature Materials, Roman Engel-Herbert, assistant professor of materials science and engineering, and his team report a new design strategy that approaches the problem from a different angle. The researchers use thin (10 nanometer) films of an unusual class of materials – called correlated metals – in which the electrons flow like a liquid. While in most conventional metals, such as copper, gold, aluminum or silver, electrons flow like a gas, in correlated metals, such as strontium vanadate and calcium vanadate, they move like a liquid. In this paper, the authors explain why these correlated metals show a high optical transparency despite their high, metal-like conductivity.
“We are trying to make metals transparent by changing the effective mass of their electrons,” Engel-Herbert says. “We are doing this by choosing materials in which the electrostatic interaction between negatively charged electrons is very large compared to their kinetic energy. As a result of this strong electron correlation effect, electrons ‘feel’ each other and behave like a liquid rather than a gas of non-interacting particles. This electron liquid is still highly conductive, but when you shine light on it, it becomes less reflective, thus much more transparent.”
To better understand how they achieved this fine balance between transparency and conductivity, they turned to a materials theory expert, Professor Karin Rabe of Rutgers University.
“We realized that we needed her help to put a number on how ‘liquid’ this electron liquid in strontium vanadate is,” Engel-Herbert says.
Rabe helped the Penn State team put together all the theoretical and mathematical puzzle pieces they needed to build transparent conductors in the form of a correlated metal. Now that they understand the essential mechanism behind their discovery, the Penn State researchers are confident they will find many other correlated metals that behave like strontium vanadate and calcium vanadate.
Lei Zhang, lead author on the Nature Materials paper and a graduate student in Engel-Herbert’s group, was the first to recognize what they had discovered.
“I came from Silicon Valley where I worked for two years as an engineer before I joined the group. I was aware that there were many companies trying hard to optimize those ITO materials and looking for other possible replacements, but they had been studied for many decades and there just wasn’t much room for improvement,” says Zhang. “When we made the electrical measurements on our correlated metals, I knew we had something that looked really good compared to standard ITO.”
Currently indium costs around $750 per kilogram, whereas strontium vanadate and calcium vanadate are made from elements with orders of magnitude higher abundance in the earth’s crust. Vanadium sells for around $25 a kilogram, less than 5percent of the cost of indium, while strontium is even cheaper than vanadium.
“Our correlated metals work really well compared to ITO. Now, the question is how to implement these new materials into a large scale manufacturing process. From what we understand right now, there is no reason that strontium vanadate could not replace ITO in the same equipment currently used in industry,” says Engel-Herbert.
Along with display technologies, Engel-Herbert and his group are excited about combining their new materials with a very promising type of solar cell that uses a class of materials called organic perovskites. Developed only within the last half dozen years, these materials outperform commercial silicon solar cells but require an inexpensive transparent conductor. Strontium vanadate, also a perovskite, has a compatible structure that makes this an interesting possibility for future inexpensive, high-efficiency solar cells.
Engel-Herbert and Zhang have applied for a patent on their technology.
Along with Zhang and Engel-Herbert, coauthors on the paper titled “Correlated metals as transparent conductors” are Hai-Tian Zhang, Craig Eaton, Yuanxia Zheng and Matthew Brahlek, all students and postdoctoral Fellows in Engel-Herbert’s group. Others from Penn State and the Materials Research Institute include Moses Chan, Evan Pugh professor of physics, and his postdoctoral Fellow Weiwei Zhao, and Venkatraman Gopalan, professor of materials science and engineering and his student Lu Guo. Rabe and her student Yuanjun Zhou, Rutgers University, and Anna Barnes, Hamna Haneef and associate professor Nikolas Podraza, University of Toledo also contributed on this project.
The Office of Naval Research, the National Science Foundation and the Department of Energy funded this work. Fabrication of the correlated metals was performed at the Materials Research Institute in the laboratory facilities of Penn State’s Millennium Science Complex.