May 26 2014
Cells have their own tiny skeletons that are responsible for many important cellular functions. EPFL scientists have developed novel fluorescent probes for imaging these important structures easily and with unprecedented resolution.
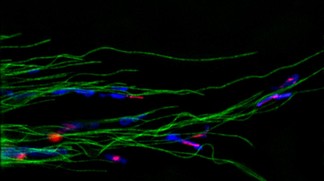 SiR-tubulin stained in green©Kai Johnsson/EPFL
SiR-tubulin stained in green©Kai Johnsson/EPFL
Like our own bodies, cells have their own skeletons called ‘cytoskeletons’ and are made of proteins instead of bones. These network-like structures maintain the cell’s shape, provide mechanical support, and are involved in critical processes of the cell’s lifecycle. The cytoskeleton is an object of intense scientific and medical research, which often requires being able to observe it directly in cells. Ideally, this would involve highly-fluorescent molecules that can bind cytoskeletal proteins with high specificity without being toxic to the cell. Publishing in Nature Methods, EPFL scientists have exploited the properties of a new fluorescent molecule, also developed at EPFL, to generate two powerful probes for the imaging of the cytoskeleton with unprecedented resolution. These probes pave the way for the easier and higher quality imaging of cells, offering many scientific and medical advantages.
The cytoskeleton is a large structure inside cells that provides them with mechanical support, keeps their three-dimensional shape and internal organization, and enables them to move and divide. It consists of three major sub-structures inside the cell, which are made up of long, filamentous proteins: tubulin and actin.
Current techniques for observing the cytoskeleton can be difficult to get into living cells, can be toxic, and are usually limited in resolution and duration, since the signal wears off over time. A common technique is fluorescence microscopy, where fluorescent molecules (‘probes’) are attached to cell structures and then ‘lit up’ against a dark background.
Staining with SiR-actin and SiR-tubulin
The team of Kai Johnsson at EPFL has developed novel fluorescent probes that can easily enter live cells, are non-toxic, have long-lasting signals, and most importantly, offer unprecedented image resolution. In 2013, the researchers developed a fluorescent molecule called silicon-rhodamine (SiR), which switches ‘on’ only when it binds to the charged surface of a protein like the ones found on the cytoskeleton. When SiR switches ‘on’, it emits light at far-red wavelengths.
The challenge was getting SiR to bind specifically to the cytoskeleton’s proteins, actin and tubulin. To achieve this, the scientists fused SiR molecules with compounds that bind tubulin or actin. The resulting hybrid molecules consist of a SiR molecule, which provides the fluorescent signal, and a molecule of a natural compound that can bind the target protein. One such compound was docetaxel, an anticancer drug that binds tubulin, and the other jasplakinolide, which specifically binds the cytoskeletal form of actin. Both compounds, which are used here in very low, non-toxic concentrations, can easily pass through the cell’s membrane and into the cell itself.
The probes, named SiR-tubulin and SiR-actin, were used to visualize the dynamics of the cytoskeleton in human skin cells. Because the light signal of the probes is emitted in the far-red spectrum, it is easy to isolate from background noise, which generates images of unprecedented resolution when used with a technique called super-resolution microscopy.
An additional advantage is the practicality of the probes. “You just add them directly into your cell culture, and they are taken up by the cells”, says Kai Johnsson. The probes also do not require any washing or preparation of the cells before administration or any subsequent washing steps, which greatly helps in maintaining the stability of their environment and their natural biological functions.
The scientists believe that they can extend their work into other types of proteins and tissues. “Cytoskeletal structures are imaged by biologists all the time”, says Johnsson. “Up to now, no probes were available that would allow you to get high quality images of microtubules and microfilaments in living cells without some kind of genetic modification. With this work, we provide the biological community with two high-performing, high-contrast fluorogenic probes that emit in the non-phototoxic part of the light spectrum, and can be even used in tissues like whole-blood samples.”
Author: Nik Papageorgiou
Source: Mediacom