Dec 2 2013
GE Healthcare, a unit of General Electric Company, introduced today the new Invenia Automated Breast Ultrasound System (ABUS) at the 2013 Radiological Society of North America (#RSNA13) annual meeting. ABUS is used to answer the need of detecting additional cancer in women where mammography alone may be insufficient due to their greater breast density.
Featuring new automated compression tools for enhanced workflow and ergonomics†, the automated breast ultrasound system provides physicians a new way to look at dense breast tissue that can allow them to improve breast cancer detection by up to 35.7 percent over mammography alone.
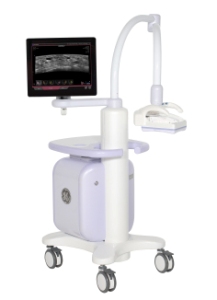 GE Healthcare stands as the sole manufacturer with an ultrasound system FDA approved for breast cancer screening. (Photo: Business Wire)
GE Healthcare stands as the sole manufacturer with an ultrasound system FDA approved for breast cancer screening. (Photo: Business Wire)
The new Invenia ABUS features advanced automation technology and is designed for reproducibility, ease of use and both patient and operator comfort. With new tools like Compression Assist and Reverse Curve, healthcare providers can quickly and comfortably capture whole breast, 3D volumes of clinical images in less time compared to previous versions of the technology. †
“Our commitment to ABUS technology and making it more clinically robust and available to more healthcare providers and patients around the world is a reflection of the company’s efforts in tackling breast cancer and our overall $1 billion investment to advance oncology solutions by 2016,” said Anders Wold, president and CEO of GE’s Ultrasound business. “The use of ultrasound technology for breast cancer screening is proving to be a valuable asset for healthcare providers in the diagnosis and care provided to patients with dense breasts.”
Dense breast tissue is a major risk factor for breast cancer and not only increases the risk of breast cancer up to four to six times, but also makes cancer more difficult to detect using mammography, according to multiple large studies. One study, published in the New England Journal of Medicine, showed mammography sensitivity is reduced by 36 to 38 percent in women with dense breasts, as density masks the appearance of tumors (Boyd, et al, NEJM 2007:356:227-36M).
To address this limitation with mammography, U-Systems (a GE Healthcare company) introduced the somo•vTM ABUS to the marketplace in September 2012 to provide healthcare providers an FDA-approved screening option for patients with dense breasts. Since the commercialization and GE’s acquisition of U-Systems, the company has continued to invest resources to determine how to further expand the reach of ultrasound technology to help improve breast care.
GE is the sole manufacturer with an FDA-approved ultrasound system for breast cancer screening purposes.
*Trademark of the General Electric Company
†Compared to the somo•vTM ABUS
GE Healthcare at #RSNA13
Each year in Chicago, the conference of the Radiological Association of North America (RSNA) provides a forum for showcasing the latest innovations in medical imaging. If you are attending the conference, please visit GE Healthcare at booth number 4033 in McCormick Place south hall. Throughout the week of the event, GE Healthcare will distribute news and information using these digital platforms:
About GE Healthcare
GE Healthcare provides transformational medical technologies and services to meet the demand for increased access, enhanced quality and more affordable healthcare around the world. GE (NYSE:GE) works on things that matter - great people and technologies taking on tough challenges. From medical imaging, software & IT, patient monitoring and diagnostics to drug discovery, biopharmaceutical manufacturing technologies and performance improvement solutions, GE Healthcare helps medical professionals deliver great healthcare to their patients.