Dec 13 2012
Chemical reactions occur so quickly that it is completely impossible to observe their progress or to control them using conventional methods. However, new developments in electrical engineering and quantum technology enable us to achieve a more exact understanding and improved control of the behaviour of atoms and molecules. At the TU Vienna, scientists have succeeded in influencing the splitting of large molecules with up to ten atoms using ultra-short laser pulses.
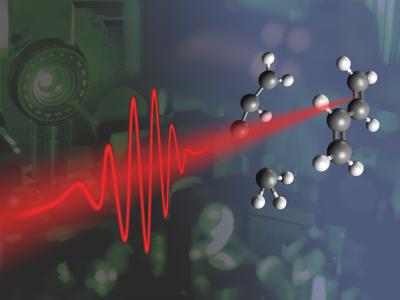 A short laser pulse hits a molecule (here: Butadiene), which splits into two parts. Credit: Vienna University of Technology
A short laser pulse hits a molecule (here: Butadiene), which splits into two parts. Credit: Vienna University of Technology
The flash of light which splits molecules
Splitting a molecule is an example of an elemental chemical reaction. The splitting of molecular bonds with a laser pulse is relatively simple. It is much more difficult, however, to influence the splitting of a specific bond in a controlled manner, i.e. to initiate it in a controlled manner or to suppress it. In order to achieve this, the complex processes must be altered at an atomic level. This is carried out at the Institute for Photonics at the TU Vienna using specially-shaped laser pulses, with a duration of only a few femtoseconds. One femtosecond (10-15 seconds) is one millionth of a billionth of a second.
Fast electrons, slow atomic nuclei
One carbon atom has a mass around 22000 times greater than an electron. It is therefore also relatively inert and cannot be moved easily from its position. A laser pulse can therefore change the movement of the small, light electrons much more rapidly than that of the atomic nuclei: One electron can be extracted from the molecule, then reversed using the laser pulse field and collided again with the molecule. During this collision, the electron can subsequently extract a second electron from the molecule. A doubly charged molecule remains, which can then split into two singly charged fragments under certain circumstances.
"Usually, it takes several femtoseconds for the atomic nuclei to reach a sufficient distance from one another and the molecule to split into two pieces", explains Markus Kitzler from the Institute of Photonics at the TU Vienna. The collision of the electron with the molecule only lasts several hundred attoseconds (10-18 seconds). "We therefore have to deal with two separate timescales", explains Kitzler. "Our specially shaped ultra-short laser pulses affect rapidly-moving electrons. The fact that the state of the electrons is changed by the collision also sets the large, slow atomic nuclei into motion."
Using this technique, the TU research team have for the first time been able to show that specific elemental chemical reactions using various hydrocarbon molecules can also be initiated or suppressed in a controlled manner, if the movement of the atomic nuclei are influenced indirectly by the much quicker electrons. The exact shape of the laser pulse is crucial in this process.
The role of electron movement for chemistry
In order to be able to interpret the experimental data correctly and understand what actually happens during these incredibly rapid processes at atomic and electronic level, theoretical calculations and computer simulations are required. This has also been carried out at the TU Vienna - at the Institute for Theoretical Physics, which collaborates with the Institute for Photonics on attosecond projects. Using this method, we can do more than simply observe whether and how a molecule splits. "The experiments and simulations show how the sequence of chemical processes can also be affected in a targeted manner using precise control of the laser pulse", explains Katharina Doblhoff-Dier from the Institute of Theoretical Physics.