Scientists of Max-Born-Institute at Germany have taken a real-time ‘movie’ through ultra-short x-ray flashes for determining spatial oscillations of valence electrons in a crystal.
 Study Using X-Ray Reaction Microscope for Spatial Oscillation Determination of Valence Electrons in Crystal
Study Using X-Ray Reaction Microscope for Spatial Oscillation Determination of Valence Electrons in Crystal
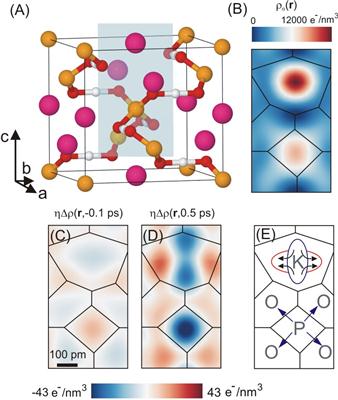
A crystal is a representation of a lattice structure, which is a well-ordered array of atoms, bonded together when electron clouds of nearby atoms interact. The inner electrons are bonded to the positively charged nuclei, whereas, the outer valence electrons are involved in the formation of chemical bonds with the neighboring atoms. The distance between the crystal’s individual atoms and its fundamental properties like electrical behavior or mechanical stability are determined by these bonds.
The atoms are continually vibrating around their equilibrium positions in the crystal lattice. A minute spatial elongation of the vibrating nuclei together with their core electron has been observed at below 1% of the distance between nearby atoms.. In order to understand the role of valence electrons for the dynamic and static electrical properties of the crystal, motion measurement of valence electrons in space and time is essential.
To study the movement of atoms and electrons in crystalline materials, an X-ray "reaction microscope" was developed by Thomas Elsaesser, Michael Woerner, Johannes Stingl, Philip Rothhardt and Flavio Zamponi.
For measurement, the ionic crystal potassium dihydrogen phosphate (KDP) vibrations are excited through an optical pulse for a time period of 50 fs. The 100 fs firm x-ray pulses that have been diffracted from the vibrations of atoms are used for measuring the momentary position of electrons and atoms. Measurement of many x-ray diffraction peaks at the same time enables reconstruction of atoms’ momentary distances and therefore, three-dimensional distribution of electrons in the crystal can be measured. The molecular movie has been developed as per stroboscope effect, where, x-ray snap shots are taken at different delay times after vibration initiation.
The scientists observed a special kind of lattice vibration, referred to as soft mode of KDP. They found that valence electrons travel 30 times more distance during oscillatory movement when compared to atoms containing nuclei and core electrons. In soft mode oscillation, the electrons move to oxygen (O) atoms from the phosphorus (P) atom, having a P-O bond length of 160 picometers, and after crossing half the oscillation period, reaches P atom again, whereas, the atoms have moved only a few picometers distance.