Dark field microscopy is a technique invented in 1830 for the observation of living, unstained cells and microorganisms. This type of microscopy requires intense illumination of the sample within a dark surrounding, making it appropriate for studying particular samples. While its limitations have seen it fall behind more modern microscopy methods, in recent years, it has gained popularity as scientists have begun to use it alongside other methods.
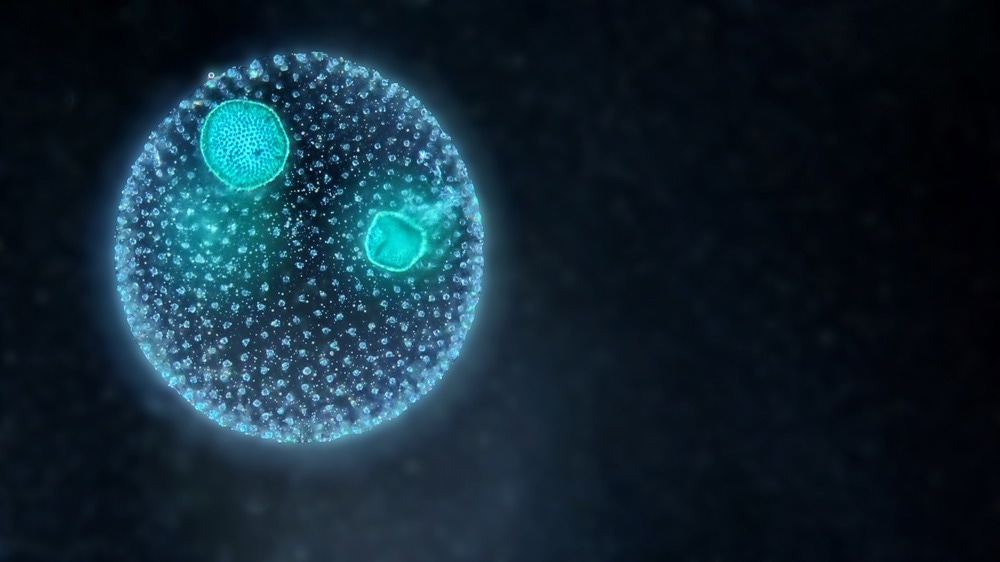
A colony of green planktonic algae Volvox under the dark field microscope. Image Credit: D. Kucharski K. Kucharska/Shutterstock.com
Principles of Dark Field Microscopy
In dark field microscopy, an opaque disk is placed beneath the condenser lens, ensuring that only light scattered from the specimen is allowed to reach the eye.
Instead of passing through the specimen, as with other forms of microscopy, in dark field, the light is reflected by particles on the slide. Light is directed so that it passes through the condenser’s outer edge at a wide angle and hits the specimen at an oblique angle.
The images usually appear as bright white against a dark background, regardless of the color of the sample. Pigments in specimens are viewed as false colors, in that the color they are depicted as in the image does not accurately reflect the actual color of the object.
Applications of Dark Field Microscopy
Dark field microscopy differs from conventional microscopy, producing different styles of images that lend themselves to different applications. Not all applications of conventional microscopy apply to dark field microscopy, and not all applications of dark field microscopy work as well with conventional techniques.
Dark field microscopy is most useful when researchers need to visualize unstained, transparent specimens. As a general rule, specimens appropriate for viewing with dark field microscopy should have a reflective index similar to the surroundings. Such a refractive index makes a sample unsuitable for viewing with conventional bright field microscopy.
As a result, viewing aquatic organisms with refractive indices similar to the surrounding water has become a standard sample studied with dark field microscopy. Other biological samples make ideal candidates for this method, such as bacteria, tissue cultures, yeast, and others. Non-biological samples suitable for study with dark force microscopy include chemical and mineral crystals and thin segments of polymers.
As a result, dark field microscopes have become an established tool in microbiology laboratories for use in various applications, including the visualization of clinical samples of spirochetes (e.g., Treponema palladium [syphilis], Borrelia burgdorferi [lyme borreliosis], and Leptospira interrogans [leptospirosis]), the observation of microbial motility (e.g., visualizing the tufts of bacterial flagella), and viewing the internal structure of eukaryotic microorganisms (e.g., algae and yeast).
Benefits and Limitations of Dark Field Microscopy
Benefits
Dark field microscopy can often image samples unsuitable for conventional bright microscopy. Compared with bright field microscopy, dark field obtains greater resolutions, thus imaging samples in greater detail.
Dark field microscopy also generates an improved image contrast vs bright field microscopy without the need to stain samples, thus, not killing cells.
Another significant benefit of dark field microscopy is that no sample preparation is required (e.g., no staining), which reduces the time demands of the process.
Finally, dark field microscopy requires no particular set-up or expensive equipment and a light microscope can be converted into a dark field microscope with minimal additional costs.
Limitations
While there are numerous essential benefits of dark field microscopy, it is not without its limitations. When visualizing wet specimens of live organisms, it is essential to do this rapidly to ensure that movement does not interfere with the quality of the image.
Next, the samples must be exposed to intense illumination, which can cause damage to the sample depending on what is being imaged.
The intensity of light required for dark field microscopy can also lead to glare and distortion. These issues also make the technique unreliable in obtaining specimen measurements.
Further limitations of dark field microscopy include its sensitivity to contaminants. Specimen slides must be meticulously cleaned to ensure that dust and dirt are eliminated from the area and such contamination can distort the images produced.
Similarly, specimens must be free of dust and air bubbles to ensure high image quality.
Finally, specimens must be thin. Dense samples can negatively impact the contrast and accuracy of images produced. For this reason, not all samples are appropriate for dark field imaging.
Final Thoughts
While dark field microscopy has been around for over a century, its limitations have hindered its ability to keep up with the vast advances in alternative microscopy techniques, such as phase contrast and differential interference contrast (DIC) microscopy. For this reason, the technique is not commonly used in modern imaging. However, it has been increasingly combined with modern methods such as fluorescence microscopy in recent years.
Read more: How the Mirrored Chip May Lead the Way to Handheld Dark-Field Microscopes
References and Further Reading
C Robert Bagnall. (2012) Dark Field Microscopy [Online]. UCI Department of Chemistry. Available at: https://www.chem.uci.edu/~dmitryf/manuals/Fundamentals/Dark%20Field%20microscopy.pdf (Last accessed October 2022).
Sekine, R., Moore, K., Matzke, M., Vallotton, P., Jiang, H., Hughes, G., Kirby, J., Donner, E., Grovenor, C., Svendsen, C. and Lombi, E. (2017) Complementary Imaging of Silver Nanoparticle Interactions with Green Algae: Dark-Field Microscopy, Electron Microscopy, and Nanoscale Secondary Ion Mass Spectrometry. ACS Nano, 11(11), pp.10894-10902. https://pubs.acs.org/doi/full/10.1021/acsnano.7b04556
Oliviero, F., Punzi, L. (2022) Basics of Polarized Light Microscopy. In: Mandell, B.F. (eds) Synovial Fluid Analysis and The Evaluation of Patients With Arthritis. Springer, Cham. https://doi.org/10.1007/978-3-030-99612-3_9
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.