Reviewed by Lexie CornerFeb 28 2024
Researchers at EPFL have created a revolutionary method that manipulates and identifies individual bacteriophages using light instead of chemical labels or bioreceptors. This could transform and expedite phage-based treatments for bacterial infections resistant to antibiotics.
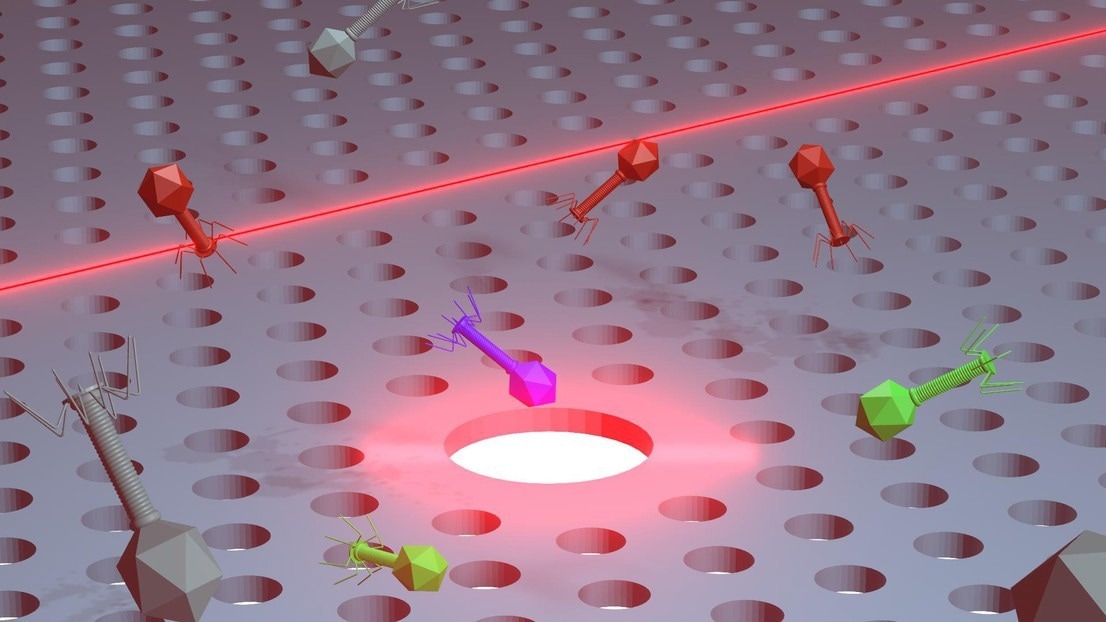 An illustration of the chip-embedded nanotweezers trapping bacteriophages. Image Credit: Nicolas Villa/EPFL
An illustration of the chip-embedded nanotweezers trapping bacteriophages. Image Credit: Nicolas Villa/EPFL
Scientists are always looking for new approaches to treating bacterial infections because antibiotic resistance poses a serious threat to human health. A potential substitute remedy could be discovered in bacteriophages, viruses that feed on bacteria, as more bacterial strains are becoming more intelligent than the medications used for decades.
The application of bacteriophages in phage treatment, an effective substitute for conventional antibiotics, is becoming increasingly popular. However, existing methods involve laborious culturing and time-consuming testing, making it difficult to discover the correct phage for a particular infection. It is like trying to find a needle in a haystack.
Researchers at EPFL, CEA Grenoble, and Lausanne University Hospital (CHUV) have created on-chip "nanotweezers" that require very little optical power to capture and manipulate individual bacteria and virions, which are infectious viruses.
The research, conducted in the EPFL group of Romuald Houdré by Nicolas Villa and Enrico Tartari, is published in the journal Small.
Nanotweezers are optical tweezers that hold and manipulate microscopic (like virions) and even sub-microscopic objects (like atoms) in three dimensions using a highly concentrated laser beam. Without physical contact, the light's gradient force draws the particles to a high-intensity focal point, "holding" them in place.
The scientist Arthur Ashkin initially created optical tweezers in 1986 after developing the underlying theories in the late 1960s. Optical tweezers continue to be highly researched, and Ashkin was awarded the 2018 Nobel Prize in Physics for his technological innovation.
Optical tweezers come in various forms. Free-space optical tweezers, for instance, may handle an object in an open environment, like liquid or air, without any structures or physical barriers to direct light. However, the researchers in this study developed optofluidic devices that combine fluidic and optical technologies on a single chip with embedded nanotweezers.
The nanotweezers, or silicon-based photonic crystal cavities, on the chip, are microscopic traps that use a force field created by light to move the phages into place gently. With the aid of the technology, the researchers could accurately manipulate individual bacteria and virions and obtain real-time data regarding the confined microorganisms.
This method differs from others in that it can differentiate between various phage species without the need for surface bioreceptors or chemical labels, which can be laborious and occasionally inefficient.
Instead, the nanotweezers “read” the distinct alterations in the characteristics of light that each particle produces to differentiate among phages. The label-free approach can greatly accelerate the selection of therapeutic phages, offering a quicker time to market for possible phage-based therapies.
The findings have consequences that go beyond phage therapy. Real-time manipulation and observation of single virions provide scientists with a potent tool for swift testing and experimentation, opening up new research directions in microbiology. In the continuous fight against infectious diseases, a fuller understanding of viruses and their interactions with hosts could result.
The research was funded by the Swiss National Science Foundation, RENATECH network (France), and the French National Research Agency (FANR).
Journal Reference:
Villa, N., et al. (2024) Optical Trapping and Fast Discrimination of Label‐Free Bacteriophages at the Single Virion Level. Small. https://doi.org/10.1002/smll.202308814.