Scientists have captured fast-moving hydrogen atoms in motion, which are essential in a vast amount of biological and chemical activities.
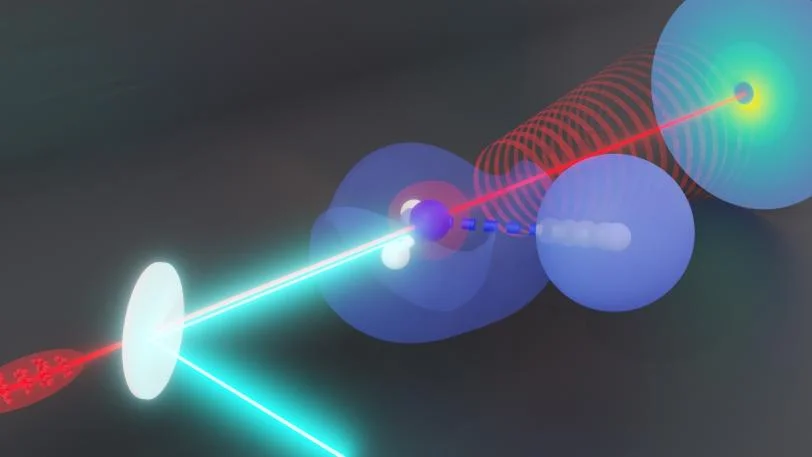 Irradiating ammonia – which is made up of one nitrogen and three hydrogens—with ultraviolet light causes one hydrogen to dissociate from the ammonia. SLAC researchers used an ultrafast “electron camera” to watch exactly what that hydrogen was doing as it dissociated. The technique had been proposed, but never proven to work, until now. In the future, researchers could use the technique to study hydrogen transfers—critical chemical reactions that drive many biological processes. Image Credit: Nanna H. List/KTH Royal Institute of Technology
Irradiating ammonia – which is made up of one nitrogen and three hydrogens—with ultraviolet light causes one hydrogen to dissociate from the ammonia. SLAC researchers used an ultrafast “electron camera” to watch exactly what that hydrogen was doing as it dissociated. The technique had been proposed, but never proven to work, until now. In the future, researchers could use the technique to study hydrogen transfers—critical chemical reactions that drive many biological processes. Image Credit: Nanna H. List/KTH Royal Institute of Technology
The mobility of hydrogen atoms within ammonia molecules was recorded using ultrafast electron diffraction (UED) by a team led by researchers from the Department of Energy’s SLAC National Accelerator Laboratory and Stanford University. Others had predicted that electron diffraction could be used to follow hydrogen atoms, but no one had done so successfully until recently.
The findings, published on October 5th, 2023 in Physical Review Letters, make use of the high-energy Megaelectronvolt (MeV) electrons’ capabilities for analyzing hydrogen atoms and proton transfers, in which the single proton that makes up the nucleus of a hydrogen atom goes from one molecule to another.
It would be beneficial to understand exactly how its structure changes during those processes since proton transfers are the driving force behind a wide variety of biological and chemical activities—think of enzymes, which aid in catalyzing biochemical reactions, and proton pumps, which are necessary for mitochondria, the powerhouses of cells.
Proton transfers, however, take just a few femtoseconds, or one millionth of one billionth of a second, to complete. It is hard to capture them in motion.
One option is to fire X-Rays at a molecule and then use the scattered X-Rays to deduce the structure of the molecule as it changes. However, X-Rays only interact with electrons; they have no effect on atomic nuclei. As a result, it is not the most accurate approach.
A group at SLAC led by scientist Thomas Wolf used MeV-UED, an ultrafast electron diffraction camera, to find the solutions they needed. They employed gas-phase ammonia, which consists of one nitrogen atom and three hydrogen atoms. The scientists used UV light to break one of the hydrogen-nitrogen bonds in ammonia. They then used an electron beam to pass through the ammonia and collect the diffracted electrons.
They not only recorded signals from hydrogen detaching from the nitrogen nucleus, but they also caught the corresponding change in the molecule’s structure. Furthermore, the scattered electrons shot at various angles, allowing them to distinguish between the two signals.
Having something that is sensitive to the electrons and something that is sensitive to the nuclei in the same experiment is extremely useful. If we can see what happens first when an atom dissociates—whether the nuclei or the electrons make the first move to separate— we can answer questions about how dissociation reactions happen.
Thomas Wolf, Lead Scientist, SLAC National Accelerator Laboratory
Scientists might use this knowledge to narrow in on the mysterious process of proton transfer, which could assist in addressing a slew of chemical and biology concerns. Understanding what protons do might have crucial ramifications in structural biology, where established technologies such as X-Ray crystallography and cryo-electron microscopy have difficulties “seeing” protons.
The researchers will repeat the experiment using X-Rays at SLAC’s X-Ray laser, the Linac Coherent Light Source (LCLS), in the future to see how different the results are. They also intend to increase the intensity of the electron beam and enhance the experiment’s temporal resolution so that they can resolve individual stages of proton dissociation over time.
The DOE Office of Science contributed to the research. MeV-UED is a SLAC LCLS X-Ray laser facility device. The LCLS is a user facility for the DOE Office of Science.
Journal Reference:
Elio, G., et al. (2023) Femtosecond Electronic and Hydrogen Structural Dynamics in Ammonia Imaged with Ultrafast Electron Diffraction. Physical Review Letters. doi:10.1103/PhysRevLett.131.143001.