The Myah device, a new optical biometer, underwent clinical validation in research published in the journal Children. The study examined its consistency in biometric measurements and agreement with the widely available optical biometer, Myopia Master.

Study: Clinical Validation of a New Optical Biometer for Myopia Control in a Healthy Pediatric Population. Image Credit: astassiya Bezhekeneva/Shutterstock.com
Global Prevalence of Myopia
Half of the world’s population is expected to be myopic in less than 30 years, with 10 percent exceeding -5 diopters. Environmental variables, such as proximity to computer screens and insufficient outdoor exposure, have been linked to the onset and progression of myopia in children.
New developments in genetic research have made it possible to identify numerous genes linked to the hereditary component of myopia. In addition, it is well-known that having severe myopia increases the risk of developing irreversible posterior segment eye diseases, such as retinal detachment and myopic maculopathy.
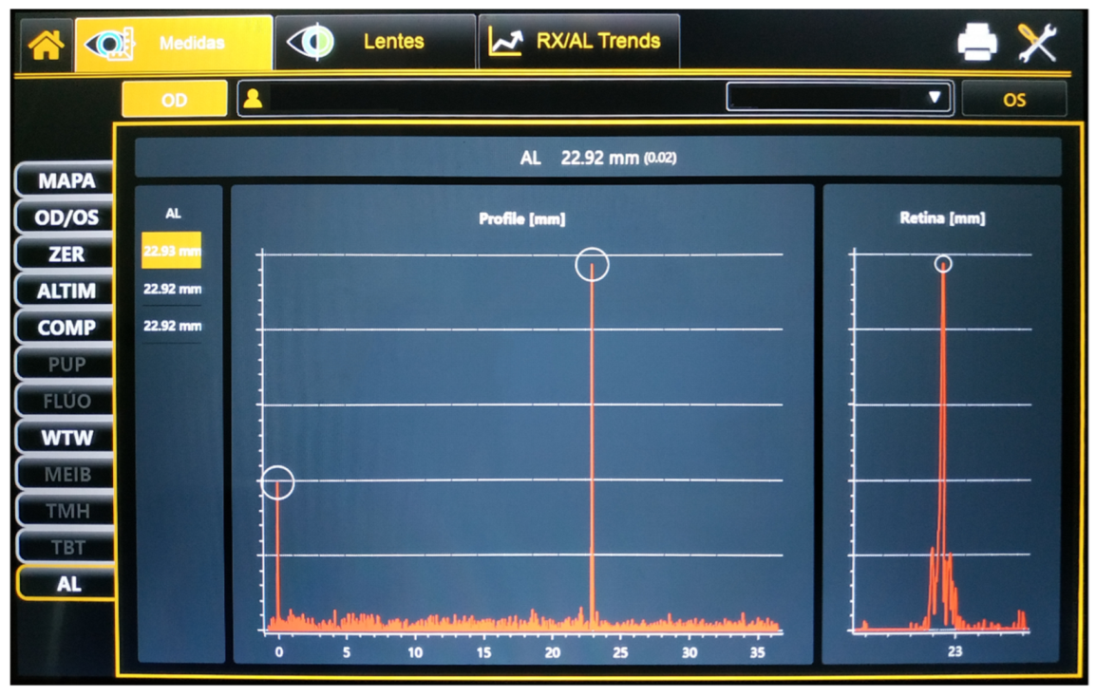
Figure 1. Display of the Myah device after an axial length measurement.
Significance of Optical Biometers in Myopia Control
Several myopia management techniques for children have been created to prevent or minimize myopia progression and its associated diseases. Lowering myopia by one diopter is expected to lessen the likelihood of developing myopic maculopathy by around 40%, emphasizing the importance of myopia control.
Myopia progression is mostly associated with an anterior-posterior axial lengthening of the ocular globe. Therefore, myopia prevention methods aim to slow the development of the refractive defect by regulating the eye’s axial length.
The 2018 FDA workshop on “Controlling the Progression of Myopia: Contact Lenses and Future Medical Devices” proposed that the axial length should be the primary target for future research and clinical trials. Therefore, ocular biometric instruments will be crucial to the investigation and treatment of myopia.
Myah® Device
Optic biometry is now possible due to the recently developed multi-diagnostic platform Myah®. The Myah device measures biometric parameters, the flat and steep corneal radius, the white-to-white distance, and the axial length. In addition, it calculates the axial length using interferometry, which is regarded as the gold standard.
Considering the importance of accurate axial length measurement for effectively controlling myopia development, clinical validation of this device is essential from a clinical standpoint.
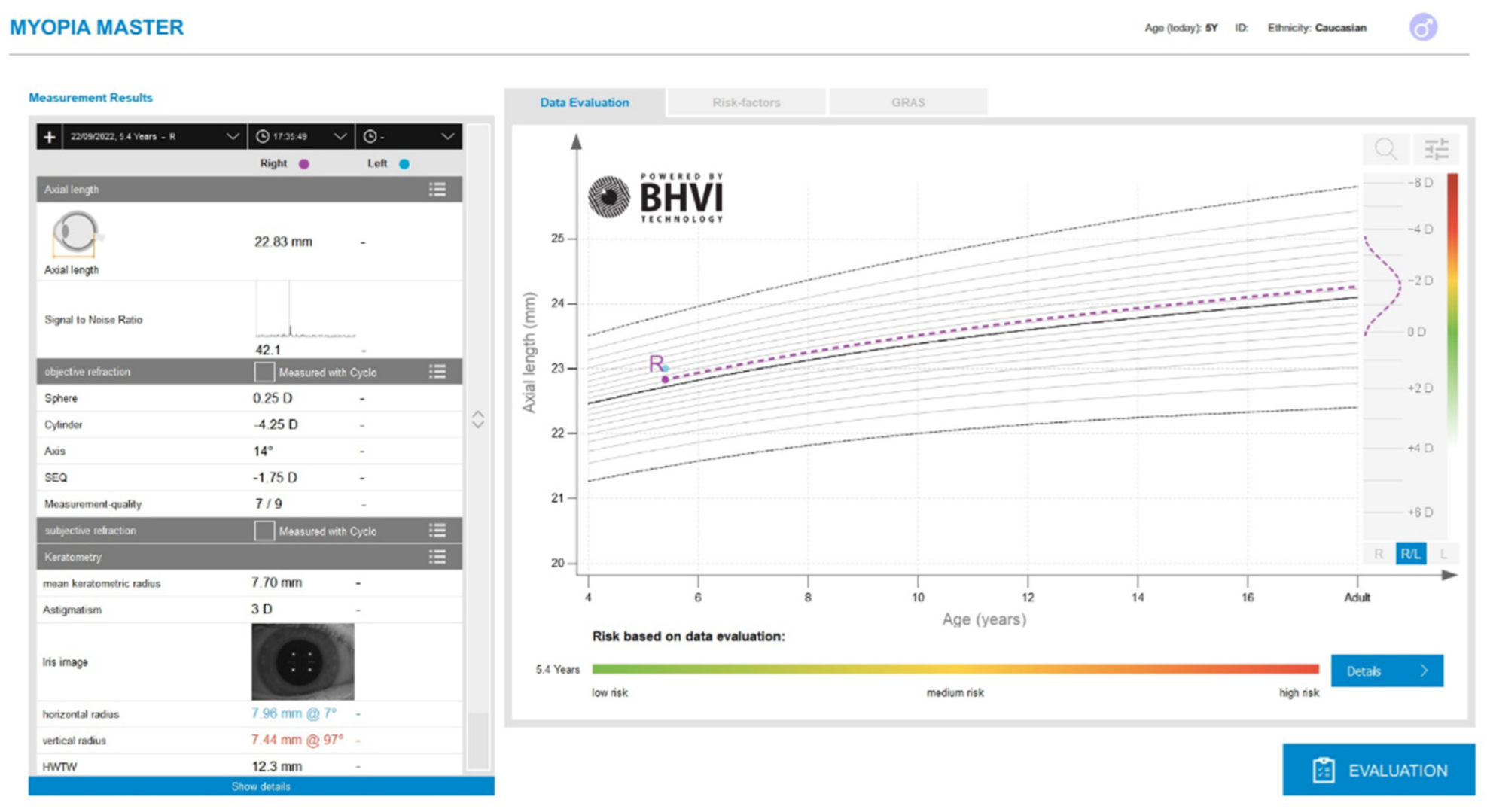
Figure 2. Display of the Myopia Master device after an optic biometric measurement.
Clinical Validation of the Myah Device in a Pediatric Population
In this study, the researchers evaluated the clinical validity of the Myah technology for the biometric parameters in a pediatric population by analyzing the repeatability of these parameters and the comparability with the Myopia Master.
51 children were enrolled for this investigation. An experienced clinician performed a comprehensive eye examination that included corrected distance visual acuity, manifest refraction, and slit-lamp assessment for both eyes.
The Myah device took repeated measurements of flat and steep corneal radius, white-to-white distance, and axial length. The same parameters were gathered from a subgroup (30 eyes) using the Myopia Master for the agreement analysis.
The reliability of the device was evaluated using the intra-class correlation coefficient and the intra-subject standard deviation. Finally, the agreement was determined using the paired Student t-test and the Bland–Altman method.
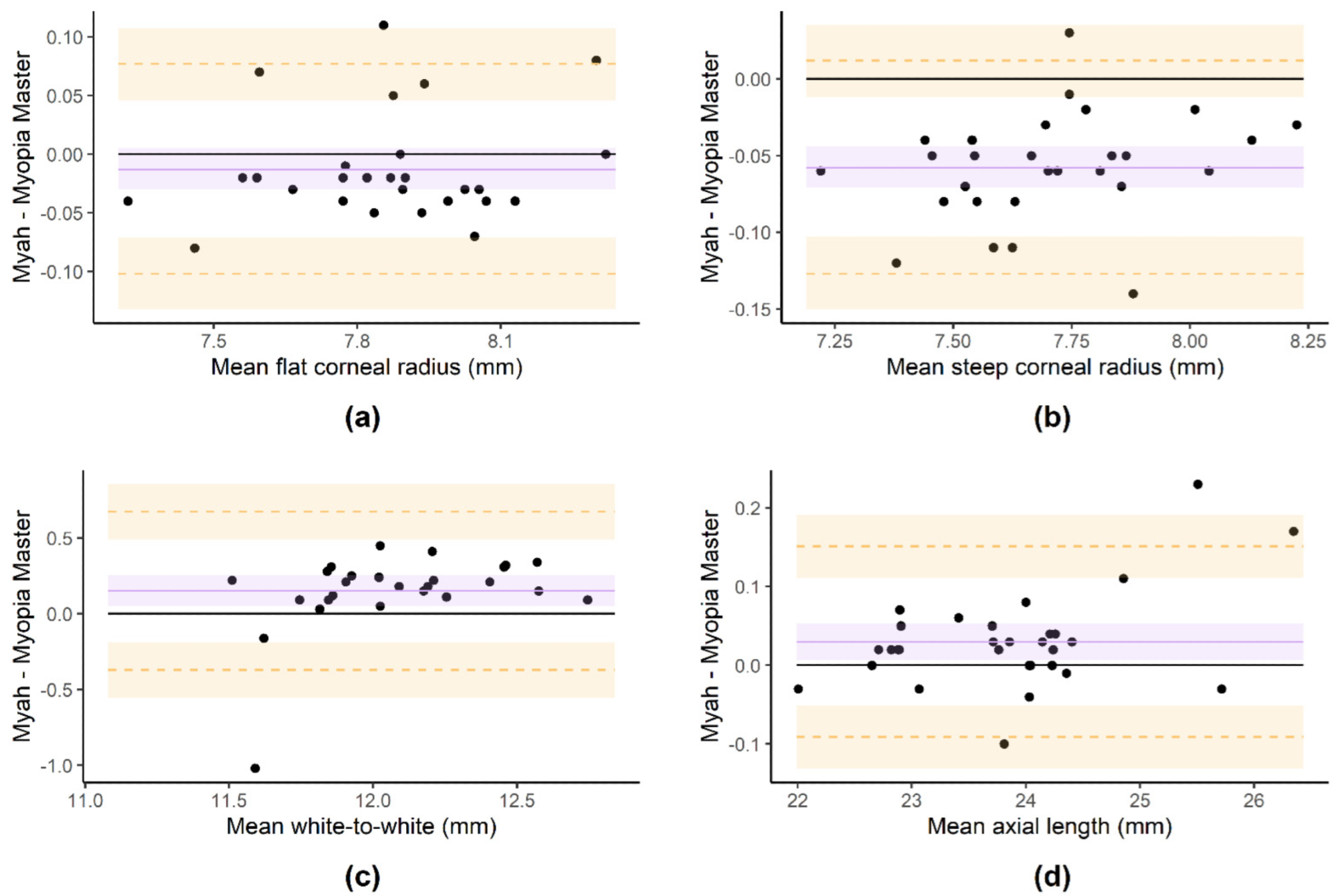
Figure 3. Bland–Altman plots of the flat corneal radius (a), steep corneal radius (b), white-to-white (c) and axial length (d) measurements performed with Myah and Myopia Master devices. The purple line and shadow represent the mean difference and its 95% confidence interval, respectively. The orange dashed lines and shadows represent the lower and upper limits of agreement and their 95% confidence intervals, respectively.
Significant Findings of the Study
The Myah device’s multiple measurements of axial length, white-to-white distance, and flat and steep corneal radius yielded intra-subject standard deviations of 0.02 mm, 0.07 mm, and 0.02 mm, 0.02 mm, respectively, with an intra-class correlation coefficient of 0.97 or more in every case.
These findings suggest that the Myah gadget reliably measures biometric parameters in a healthy pediatric population. Therefore, eye care professionals involved in myopia control can confidently use the Myah device to evaluate corneal geometry and myopia progression, especially in children who require contact lenses for myopia control.
The Myopia Master and Myah devices demonstrated excellent correlation for the corneal radius readings, while the agreement for the other two biometric parameters is clinically acceptable. Therefore, in a healthy pediatric population, the Myah device’s measurements of biometric parameters could be used instead of the Myopia Master device’s measurements, but with some caution.
Limitations
Although just two devices were chosen to fulfill the study’s objectives, the market today offers a variety of biometers. The Myopia Master and the Myah devices were chosen for comparison because both were designed especially to measure axial development, which is particularly important in the current pediatric population.
Given the growing interest in myopia correction, the clinical validation was conducted on a pediatric population. However, evaluation of the Myah device in an older population would enhance our understanding of the instrument’s effectiveness.
For biometric assessments, the absence of cycloplegia might be considered a constraint. However, it has already been reported that cycloplegia does not affect corneal curvature or axial length measurements conducted with various biometers in pediatric and adult populations.
Reference
Martínez-Plaza, E., Molina-Martín, A., Arias-Puente, A., & Piñero, D.P. (2022). Clinical Validation of a New Optical Biometer for Myopia Control in a Healthy Pediatric Population. Children. https://www.mdpi.com/2227-9067/9/11/1713/htm
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.