Reviewed by Alex SmithAug 18 2022
Researchers have created a label-free, non-invasive Raman spectroscopy method that can capture microscopic images of biological specimens promptly and accurately identify a diversity of biomolecules.
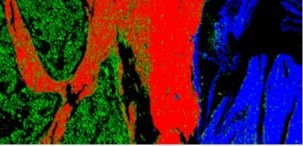 The researchers used their CARS microscopy system to image a tissue sample from a mouse spine. The image shows the bone marrow region (green), muscle region (blue) and the bone tissue (red). Image Credit: Dario Polli, Politecnico di Milano
The researchers used their CARS microscopy system to image a tissue sample from a mouse spine. The image shows the bone marrow region (green), muscle region (blue) and the bone tissue (red). Image Credit: Dario Polli, Politecnico di Milano
Our work could lead to a non-invasive, label-free, and user-friendly device for clinical use. This innovative microscope, coupled with deep learning-based algorithms, could eventually make it easier and faster to diagnose cancer by allowing the visualization of the chemical constituents of human tissues and cells.
Dario Polli, Research Team Leader, Politecnico di Milano
The researchers disclosed their new mechanism, which is centered on coherent anti-stokes Raman scattering (CARS) microscopy, in the Optica Publishing Group’s journal Optics Express. CARS microscopy takes advantage of the interaction between ultra-short laser pulses and biological samples to create images based on the vibrational signatures of molecules.
The new method allows access to the 400–1800 cm–1 fingerprint region, a difficult-to-detect part of the vibrational spectrum. Although many particular substances in this region can be recognized by their vibrational signatures, it typically produces faint signals that are difficult to identify.
Polli added, “Commonly used techniques in biomedical sciences often require staining, which is not only cumbersome but can also introduce structural and chemical alterations that can lead to artifacts, or errors, in imaging and data processing. Because our system can distinguish between many different chemical species in biological tissues without labels, it could be useful for live cell imaging and analyzing tissue biopsies.”
Lower Repetition Rate, Faster Imaging
This new research is a part of the European Commission-funded CRIMSON project, which aims to create a turnkey imaging tool that employs vibrational spectroscopy for quick cell and tissue classification. The project’s objective is to revolutionize the study of the molecular origins of diseases to make way for fresh ideas that might progress personalized medicine.
The researchers created a CARS microscope premised on a commercial laser that generates ultrashort pulses with lengths of about 270 femtoseconds in the near-infrared wavelength region as a significant step toward this goal.
They chose to use laser pulses with a repetition rate of 2 MHz for the microscopy system as opposed to the 40 or 80 MHz employed by the majority of other CARS systems.
Since there is a 0.5-microsecond delay between two successive pulses, the sample is less likely to sustain photothermal damage when the repetition rate is reduced. Along with producing a stronger CARS signal and a quicker acquisition speed, it also results in higher pulse energy and peak intensity at the focus point.
The most important advantage of the lower repetition rate is that it allowed us to generate broadband, red-shifted Stokes pulses that cover the whole fingerprint vibrational region by using white-light supercontinuum generation in a bulk crystal. Compared to other methods, this approach is technically simpler, more compact, and robust.
Federico Vernuccio, Study First Author and Doctoral Student, Politecnico di Milano
Higher laser intensities can be used prior to the commencement of photodamage by employing a spectral area that is red-shifted in comparison to conventional settings. The researchers also created brand-new algorithms that integrate AI and conventional numerical computational techniques.
These algorithms extract more information from the acquired data and transform it into images that make it easy to differentiate between various chemical species.
Vernuccio stated, “Thanks to our improvements, the CARS system delivers high-quality images at a state-of-the-art acquisition speed. Our system has a less than 1-millisecond pixel dwell time without compromising sample integrity. This speed is limited by the spectrometer refresh rate.”
High Speed Sensitivity
The researchers utilized reference samples to compare the spectra obtained with the new microscope and those obtained using an advanced but slower vibrational spectroscopy technique to test their system.
The remarkable agreement between the two techniques proved that the new system was capable of delivering spectra at very high rates with good spectral resolution and chemical specificity.
The researchers subsequently obtained CARS spectra of a series of dimethyl sulfoxide solutions with varying concentrations to evaluate the detection limit of their method. The system’s unmatched sensitivity of 14.1 mmol/liter, or roughly double that of existing CARS systems operating in the same region, allowed it to quantify chemical concentration.
They also demonstrated how the system could discriminate and localize different transparent micron-sized plastic beads based on their vibrational signatures, and they measured biological tissues to show that the method is effective on biological samples without causing harm.
“Our CARS microscope allows label-free imaging with chemical specificity at higher speeds, thus making Raman imaging of living cells more feasible. This could allow our system to be used to analyze the interactions of cancer cells with immune cells or to characterize how chemotherapy affects cells, for example,” Polli further stated.
With the support of white-light supercontinuum generation, the researchers are currently attempting to enhance their system by generating Stokes pulses across an even wider wavelength range.
This would increase the number of chemical analytes that could be detected as well as the speed of imaging. They are also working toward commercialization by designing compact optical sources, user-friendly software, and designs for a commercial prototype and detection system.
Journal Reference:
Vernuccio, F., et al. (2022). Fingerprint multiplex CARS at high speed based on supercontinuum generation in bulk media and deep learning spectral denoising. Optics Express. doi:10.1364/OE.463032