Microscopes are considered to be a useful tool in biomedical research as they enable the close examination and visualization of tissues. Due to the opaque nature of biological materials, light scattering is severe when it passes through tissues, resulting in a high amount of background noise and complicated optical distortion.
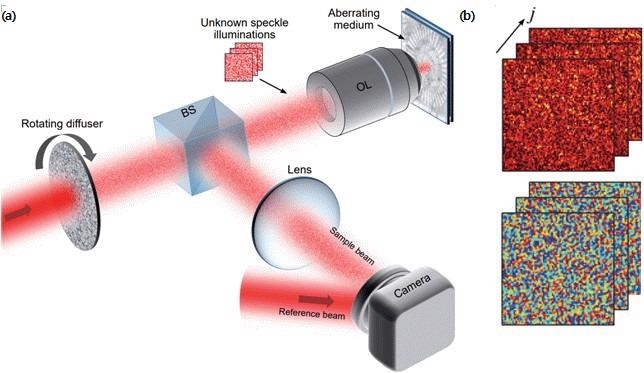 (a) Schematic of compressed time-reversal matrix microscope. Random speckle fields generated by a rotating diffuser sequentially illuminate the sample underneath an aberrating medium, and the reflected speckle fields are measured by an off-axis digital holographic microscope. BS: beam splitter. OL: objective lens. (b) Examples of measured hologram images for speckle fields. (top: intensity, bottom: phase). Image Credit: Institute for Basic Science.
(a) Schematic of compressed time-reversal matrix microscope. Random speckle fields generated by a rotating diffuser sequentially illuminate the sample underneath an aberrating medium, and the reflected speckle fields are measured by an off-axis digital holographic microscope. BS: beam splitter. OL: objective lens. (b) Examples of measured hologram images for speckle fields. (top: intensity, bottom: phase). Image Credit: Institute for Basic Science.
Most light microscopes only allow users to see the tissue surface, and many microscopes are incapable of seeing features that are numerous cell layers deep. However, obtaining high-resolution optical pictures of microstructures deep within tissues is extremely difficult.
About a year ago, a research team headed by Professor Wonshik Choi from the Institute of Basic Science’s Center for Molecular Spectroscopy and Dynamics (CMSD) demonstrated “reflection matrix microscopy,” an imaging technique that merged the capabilities of both hardware and computational advanced adaptive optics.
It analyzes a reflection matrix, which comprises all available information regarding the connection between the input and output fields of an imaging system, especially objects of interest, in contrast to traditional imaging.
Post-digital image processing can however retrieve a crisp, unaltered image of the object from the measured matrix.
As a result, the technology has emerged as a viable option for non-invasive, high-resolution optical imaging deep within biological tissues without the use of labels. The majority of the traditional AOs are outperformed by matrix imaging. The researchers showed that their method could “see through” an undamaged mouse skull and enable the precise imaging of neurons beneath it.
Regardless of its outstanding performance, reflection matrix microscopy had some flaws. Since a significant number of interferometric pictures for all available input illumination fields must be measured, monitoring the whole reflection matrix is time-consuming and susceptible to extraneous disruptions.
While sparser sampling can accelerate the process, inadequate sampling can restrict the capacity to rectify distortions. As a result, actual volumetric imaging of living samples was not achievable, posing practical limits in its implementation to biodynamic studies.
The very same IBS group recently launched a new and enhanced version of their prior AO microscope technology in a study published in the journal Light: Science & Applications. This revolutionary real-time volumetric AO imaging method enables 3D imaging of strongly aberrated samples over a large depth range while minimizing picture deterioration.
Choi’s team used compressed sensing in the framework of matrix imaging to expedite data collecting. To progressively expose unknown speckle patterns on a sample, researchers simply added a revolving optical diffuser to their already deployed reflection matrix microscope.
Furthermore, they generated a compressed reflection matrix by acquiring a substantially fewer number of speckle photos than was previously necessary, resulting in a nearly 100-fold reduction in matrix acquisition time.
Researchers used a compressed so-called “time-reversal matrix” and a novel technique to detect sample information and distortions individually in image post-processing. This technique is capable of not only drastically reducing matrix acquisition time but also eliminating the necessity for precise calibration and illumination pattern determination.
Volumetric AO imaging can be done in almost live time using compressed time-reversal matrix imaging. The abilities of the new microscope were proven in a mouse brain by aberration-free 3D imaging of myelin nerve fibers. For volumetric imaging of 128 × 128 × 125 μm3 tissue, the data acquisition time was only 3.58 seconds, having an axial resolution of 2 μm and diffraction-limited lateral resolution of 0.45 μm.
It is predicted to pave the way for realistic matrix imaging applications in all domains of wave engineering, especially biomedical imaging.
Faster reflection matrix imaging technology is expected to enable real-time, nondestructive 3D optical diagnosis in the future, which will lead to faster diagnosis and advances in neuroscience research. We will further develop it to broaden the scope of its application in all wave engineering disciplines, including biomedical imaging.
Wonshik Choi, Associate Director and Professor, Center for Molecular Spectroscopy and Dynamics, Institute of Basic Science
Journal Reference:
Lee, H., et al. (2022) High-throughput volumetric adaptive optical imaging using compressed time-reversal matrix. Light: Science & Applications. doi.org/10.1038/s41377-021-00705-4.