Reviewed by Alex SmithNov 26 2021
Solid-solution organic crystals are being used in the quest for better photon upconversion materials, which convert currently wasted long-wavelength light into more beneficial shorter wavelength light.
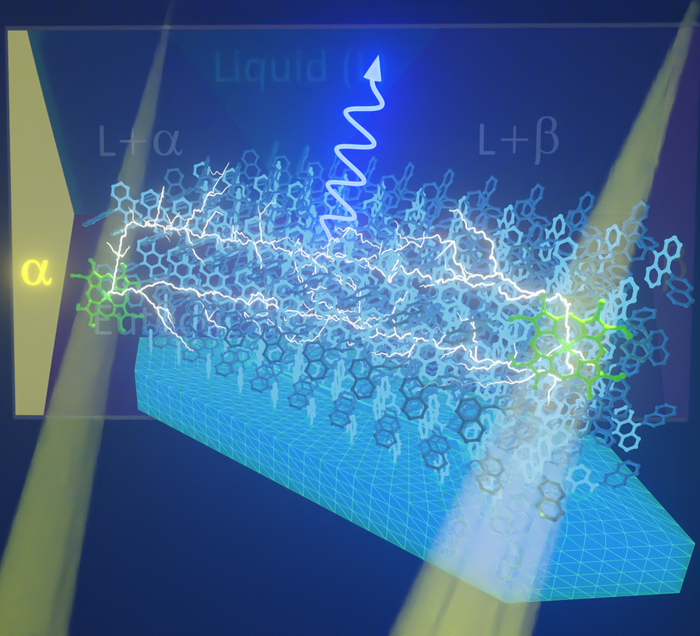 Image of photon upconversion using a developed crystal through triplet-triplet annihilation. The sensitizer molecules (green) absorb low-energy photons (long-wavelength light) and become excited into triplet states. These triplet states are then transferred to nearby annihilator molecules (blue), which then pass them around throughout the crystalline array of the annihilator. If two traveling triplet states meet at a single annihilator molecule, the combined excess energy produces a higher energy.
Image of photon upconversion using a developed crystal through triplet-triplet annihilation. The sensitizer molecules (green) absorb low-energy photons (long-wavelength light) and become excited into triplet states. These triplet states are then transferred to nearby annihilator molecules (blue), which then pass them around throughout the crystalline array of the annihilator. If two traveling triplet states meet at a single annihilator molecule, the combined excess energy produces a higher energy.
Researchers from the Tokyo Institute of Technology reconsidered a materials method formerly considered uninspiring—using a molecule initially created for organic LEDs—accomplishing exceptional performance and efficiency. Their findings open doors to many unique photonic technologies, such as improved solar cells and photocatalysts for hydrocarbon and hydrogen manufacture.
Light is a robust source of energy that can, if exploited correctly, be used to stimulate rigid chemical reactions, produce electricity, and work optoelectronic devices. But, in a majority of applications, not all the wavelengths of light can be employed.
The reason is that the energy that each photon holds is inversely proportional to its wavelength, and physical and chemical processes are activated by light only when the energy provided by individual photons surpasses a specific threshold.
This means that devices such as solar cells cannot gain from all the color found in sunlight, as it contains a blend of photons with both low and high energies. Researchers around the world are actively examining materials to establish photon upconversion (PUC), by which photons possessing lower energies (longer wavelengths) are trapped and re-emitted as photons with higher energies (shorter wavelengths).
One favorable way to achieve this is via triplet-triplet annihilation (TTA). This process necessitates the integration of a sensitizer material and an annihilator material. The sensitizer absorbs low-energy photons (long-wavelength light) and shifts its excited energy to the annihilator, which produces higher energy photons (light of shorter wavelength) because of TTA.
Discovering beneficial solid materials for PUC has been a tough challenge for a long time. Although liquid samples can realize comparatively high PUC efficiency, experimenting with liquids, particularly those containing organic solvents, is inherently hazardous and laborious in various applications.
However, preceding trials to develop PUC solids mostly suffered from weak crystal quality and small crystal domains, which resulted in short traveling distances of triplet excited states and therefore, low PUC efficiency.
Furthermore, in earlier solid PUC samples, stability under nonstop photoirradiation was not verified, and experimental data were frequently attained in inert gas atmospheres. Therefore, the low efficiency and inadequate materials stability had been an issue for a long time.
At present, in a new study headed by Associate Professor Yoichi Murakami from Tokyo Tech, Japan, a team of scientists discovered the answer to this issue. As reported in Materials Horizon, their study (open access) illustrates how they concentrated on van der Waals crystals, a classical materials group that has not been explored for the search of high-efficiency PUC solids.
After realizing that 9-(2-naphthyl)-10-[4-(1-naphthyl)phenylanthracene (ANNP), a hydrocarbon molecule initially created for blue organic LEDs, was an outstanding annihilator for embodying their concept, they tried blending it with platinum octaethylporphyrin (PtOEP), a staple sensitizer that captures green light.
The researchers learned that aggregation of the sensitizer molecules could be fully avoided by exploiting the crystalline phase of a van der Waals solid solution with an adequately low proportion of PtOEP to ANNP (around 1:50000). They continued to methodically define the obtained crystals and gained some insight into why using the ANNP annihilator stopped the aggregation of the sensitizer when other current annihilators had been unsuccessful in doing so in earlier studies.
Besides, the solid crystals produced by the researchers were very stable and showed exceptional performance.
The results of our experiments using simulated sunlight indicate that solar concentration optics such as lenses are no longer needed to efficiently upconvert terrestrial sunlight.
Yoichi Murakami, Study Lead and Associate Professor, Tokyo Tech
Generally, this study reintroduces van der Waals crystals into the game of PUC as an effective approach to developing exceptional solid materials using flexible hydrocarbon annihilators.
The proof-of-concept we presented in our paper is a major technical leap forward in the quest for high-performance PUC solids, which will open up diverse photonics technologies in the future.
Yoichi Murakami, Study Lead and Associate Professor, Tokyo Tech
Further research on this subject would allow us to efficiently alter light into its most beneficial forms.
Journal Reference:
Enomoto, R., et al. (2021) Van der Waals solid solution crystals for highly efficient in-air photon upconversion under subsolar irradiance. Materials Horizons. doi.org/10.1039/D1MH01542Ga.