May 4 2020
The preliminary step in a majority of light-driven chemical reactions, such as the ones that drive human vision and photosynthesis, is a change in the arrangement of the electrons of a molecule as these electrons capture the light’s energy.
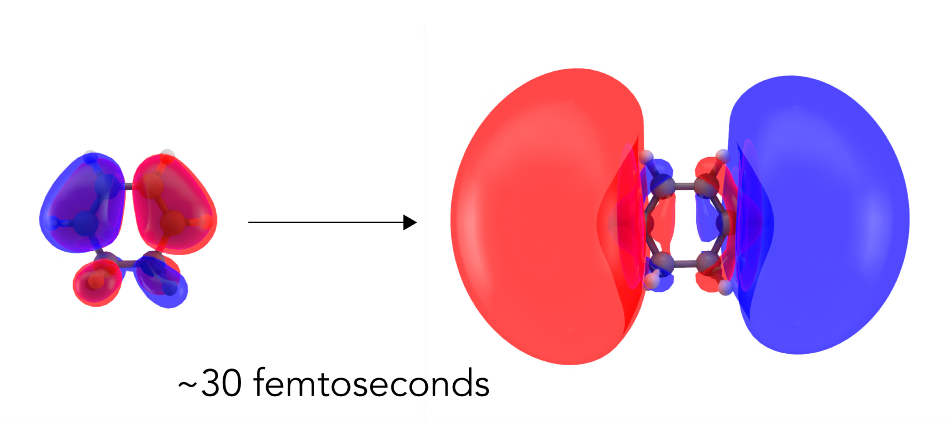 Scientists have directly seen the first step in a light-driven chemical reaction for the first time. They used an X-ray free-electron laser at SLAC to capture nearly instantaneous changes in the distribution of electrons when light hit a ring-shaped molecule called CHD. Within 30 fs, or millionths of a billionth of a second, clouds of electrons deformed into larger, more diffuse clouds corresponding to an excited electronic state. Image Credit: Greg Stewart/SLAC National Accelerator Laboratory.
Scientists have directly seen the first step in a light-driven chemical reaction for the first time. They used an X-ray free-electron laser at SLAC to capture nearly instantaneous changes in the distribution of electrons when light hit a ring-shaped molecule called CHD. Within 30 fs, or millionths of a billionth of a second, clouds of electrons deformed into larger, more diffuse clouds corresponding to an excited electronic state. Image Credit: Greg Stewart/SLAC National Accelerator Laboratory.
This slight rearrangement of the electrons precedes the steps for everything that follows and establishes the way the reaction continues.
Now for the first time, researchers have directly observed this preliminary step, visualizing how the electron cloud of the molecule, balloons out before the response of any of the atomic nuclei present in the molecule.
Although such a response has been estimated theoretically and identified indirectly, this is the first time where it has been directly captured with X-rays in a process called molecular movie-making. The ultimate objective of this process is to view the real-time behavior of both nuclei and electrons during the formation or breakage of chemical bonds.
A research team, from the University of Edinburgh, Brown University, and SLAC National Accelerator Laboratory at the Department of Energy (DOE), has recently reported its discoveries in the Nature Communications journal.
In past molecular movies, we have been able to see how atomic nuclei move during a chemical reaction. But the chemical bonding itself, which is a result of the redistribution of electrons, was invisible. Now the door is open to watching the chemical bonds change during reactions.
Peter Weber, Study Senior Author and Chemistry Professor, Brown University
A Model for Important Biological Reactions
This was the newest in a sequence of molecular movies featuring 1,3-cyclohexadiene (CHD)—a ring-shaped molecule obtained from pine oil. In a low-pressure gas condition, CHD’s molecules float freely and can be analyzed easily. CHD also acts as a crucial model for more intricate biological reactions similar to the one that creates vitamin D when sunlight touches the skin.
In studies dating back to nearly two decades, researchers have explored how the ring of the CHD breaks down when bombarded with light—initially with electron diffraction methods, and lately with SLAC’s “electron camera,” MeV-UED, as well as the X-ray free-electron laser called Linac Coherent Light Source (LCLS). These studies along with other analyses conducted worldwide have demonstrated the way the reaction proceeds in increasingly finer detail.
Four years ago, LCLS was used by the scientists from SLAC National Accelerator Laboratory, Brown University, and the University of Edinburgh to develop a molecular movie in which the CHD ring is flying apart—the first-of-its-kind molecular movie to be captured through X-rays. Such an achievement was regarded as one of the 75 most significant scientific innovations to arise from a DOE national laboratory, together with the findings like DNA decoding and neutrino detection.
However, those earlier experiments were unable to view the preliminary electron-shuffling step, since no appropriate way was available to pull it apart from the relatively larger movements of the atomic nuclei of the molecule.
Electrons in the Spotlight
For this analysis, Weber headed an experimental team that adopted a somewhat different method: The researchers bombarded the CHD gas samples with a wavelength of laser light that activated the molecules into a state that lasts for a comparatively long period of time—that is, 200 fs, or millionths of a billionth of a second—thus allowing their electronic structure to be examined with LCLS X-ray laser pulses.
X-ray scattering has been used to determine the structure of matter for more than 100 years, but this is the first time the electronic structure of an excited state has been directly observed.
Adam Kirrander, Study Senior Co-Author and Senior Lecturer, Edinburgh University
The method employed, known as non-resonant X-ray scattering, quantifies the electrons’ arrangement in a sample, and the researchers are hoping to record these changes in the electrons’ distribution when the light is absorbed by the molecule.
The researchers’ measurement bore out that prediction—while the electrons’ signal was feeble, the team was able to explicitly record the way the electron cloud distorted into a bigger and more diffuse cloud that matches with an activated electronic state. It was important to view these changes in the electrons before the nuclei begin to move.
In a chemical reaction, the atomic nuclei move and it’s difficult to disentangle that signal from the other parts that belong to chemical bonds forming or breaking. In this study, the change in the positions of atomic nuclei is comparatively small on that timescale, so we were able to see the motions of electrons right after the molecule absorbs light.
Haiwang Yong, PhD Student and Study Lead Author, Brown University
Michael Minitti, a SLAC senior staff scientist, added, “We’re imaging these electrons as they move and shift around. This paves the way to watching electron motions in and around bond breaking and bond formation directly and in real time; in that sense it’s similar to photography.”
Nikola Zotev, a PhD student in Kirrander’s laboratory at the University of Edinburgh, played an important role in the modeling and computational work in this research. LCLS is a DOE Office of Science user facility. The Office of Science and the Carnegie Trust for the Universities of Scotland have mainly funded the study.