Feb 7 2020
Photosensitizers can be described as molecules with the ability to absorb sunlight and transfer that energy to drive chemical reactions or produce electricity.
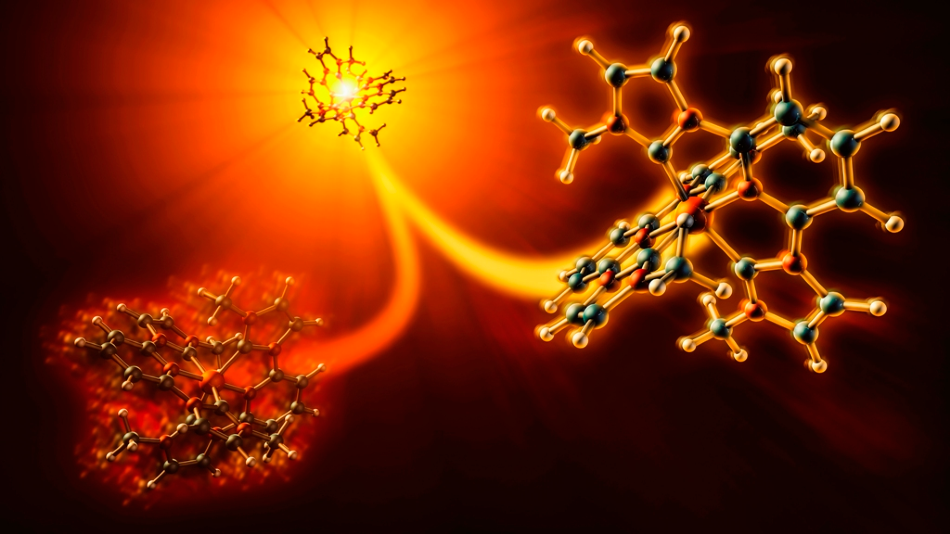 Experiments at SLAC showed that an inexpensive photosensitizer molecule, iron carbene, can respond in two competing ways when hit by light. Only one of those pathways (right) allows electrons to flow into devices or chemical reactions where they’re needed. The molecules took this energy-producing path about 60% of the time. Image Credit: Greg Stewart/SLAC National Accelerator Laboratory.
Experiments at SLAC showed that an inexpensive photosensitizer molecule, iron carbene, can respond in two competing ways when hit by light. Only one of those pathways (right) allows electrons to flow into devices or chemical reactions where they’re needed. The molecules took this energy-producing path about 60% of the time. Image Credit: Greg Stewart/SLAC National Accelerator Laboratory.
Most often, the photosensitizers are made of rare, high-cost metals. Therefore, in the last few years, the finding that iron carbenes, which include plain old iron at their cores, have the ability to act as photosensitizers. This has led to several research studies focused on this area.
Although iron carbenes that are more efficient like never before are being discovered, researchers must gain insights into the precise behavior of these molecules at an atomic level, to be able to design them for ultimate performance.
At the Department of Energy’s SLAC National Accelerator Laboratory, scientists have now used an X-ray laser to observe what occurs when an iron carbene is hit by light. They found that iron carbene can respond in two conflicting ways. Of these ways, only one permits electrons to flow into the devices or reactions where they are in demand.
In this experiment, the molecule obeyed the energy-generating path nearly 60% of the time. The study outcomes were reported in Nature Communications on January 31st, 2020.
An international team led by scientists from the Stanford PULSE Institute at SLAC endeavored to find how this works by investigating iron carbene samples using X-ray laser pulses from the Linac Coherent Light Source (LCLS) at the lab. They concurrently measured two different signals that show how the atomic nuclei of the molecule move and how its electrons move in and out of the iron-carbene bonds.
From the results, it was observed that the electrons occupied the carbene attachments for a good amount of time to do useful work nearly 60% of the time. The rest of the time, they went back to the iron atom very soon, without achieving anything.
According to Kelly Gaffney from PULSE, the long-term objective of this study is to make close to 100% of the electrons to bind to the carbenes for a longer time, to enable the use of energy from light to trigger chemical reactions. To achieve this, researchers must find design principles for customizing iron carbene molecules to perform specific jobs with ultimate efficiency.
Kristjan Kunnus, a postdoctoral researcher at PULSE, headed the analysis for this research, which was performed at LCLS and at SLAC’s Stanford Synchrotron Radiation Lightsource (SSRL), both DOE Office of Science user facilities.
Scientists at Lund University in Sweden prepared the samples for analysis. Researchers from Uppsala University in Sweden, Technical University of Denmark, Copenhagen University, Wigner Research Centre for Physics and ELI-ALPS, ELI-HU Non-Profit Ltd in Hungary, and Deutsches Elektronen-Synchrotron (DESY) in Germany also contributed to the study. The DOE Office of Science provided a major part of the financial support for this study.