Jan 20 2020
Gastrointestinal (GI) disorders can be diagnosed, treated, or monitored by inserting a wide range of medical devices into the GI tract.
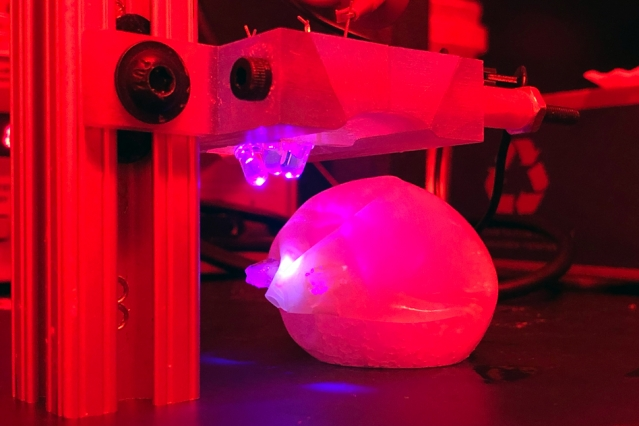 MIT engineers demonstrated a bariatric balloon that can be inflated in the stomach and then degraded by shining light on the seal, which is made of a novel light-sensitive polymer. Image Credit: Ritu Raman.
MIT engineers demonstrated a bariatric balloon that can be inflated in the stomach and then degraded by shining light on the seal, which is made of a novel light-sensitive polymer. Image Credit: Ritu Raman.
A majority of these devices have to be taken out through an endoscopic procedure as soon as their job is over. But engineers at the Massachusetts Institute of Technology (MIT) have now developed a new method to activate the medical devices to disintegrate within the body when they are subjected to light emitted by an ingestible LED.
The latest method is based on a novel light-responsive hydrogel developed by the scientists. Medical devices integrated with this hydrogel can prevent several endoscopic surgeries and would provide physicians with an easier and quicker way to remove medical devices when they are not working properly or not needed anymore, stated the scientists.
We are developing a set of systems that can reside in the gastrointestinal tract, and as part of that, we’re looking to develop different ways in which we can trigger the disassembly of devices in the GI tract without the requirement for a major procedure.
Giovanni Traverso, Study Senior Author and Assistant Professor, Department of Mechanical Engineering, MIT
Traverso is also a gastroenterologist at Brigham and Women’s Hospital.
By studying pigs, the scientists demonstrated that medical devices produced from the light-responsive hydrogel could be stimulated to disintegrate after it is subjected to ultraviolet light or blue light emitted by a tiny LED.
Ritu Raman is a postdoc at the Koch Institute for Integrative Cancer Research at MIT and is the study’s lead author. The study was recently published in the Science Advances journal.
Other authors who contributed to the study are technical associates Declan Gwynne, Joy Collins, and Siddartha Tamang; former technical associates Tiffany Hua, Jianlin Zhou, Tina Esfandiary, and Vance Soares; graduate student Simo Pajovic; Division of Comparative Medicine veterinarian Alison Hayward; as well as David H. Koch Institute Professor Robert Langer.
Controlled Breakdown
Traverso and Langer have created several ingestible devices in the last few years. These devices were developed to stay in the GI tract for long periods of time. The duo has also worked on many different approaches to regulate the breakdown of these ingestible devices, such as exposure to specific chemicals, or techniques based on variations in temperature or pH.
Given our interests in developing systems that can reside for prolonged periods in the gastrointestinal tract, we continue to investigate a range of approaches to facilitate the removal of these systems in the setting of adverse reaction or when they are no longer needed. We’re really looking at different triggers and how they perform, and whether we can apply them to different settings.
Giovanni Traverso, Study Senior Author and Assistant Professor, Department of Mechanical Engineering, MIT
In the latest study, the scientists investigated a light-based trigger, which according to them can provide certain benefits over their previous methods. One possible benefit is that light can work at a distance and does not have to make direct contact with the material being disintegrated. Moreover, light usually does not enter into the GI tract, and hence, accidental triggering is not likely to occur.
Raman created a light-responsive hydrogel to develop the novel material. The hydrogel is based on a material designed in the laboratory of Kristi Anseth, a former postdoc in Langer laboratory and currently a professor of chemical and biological engineering at the University of Colorado at Boulder.
The polymer gel features a chemical bond that breaks down upon exposure to a wavelength of light from 405 to 365 nm (blue to ultraviolet).
Rather than producing a material that is exclusively made up of that light-responsive polymer, Raman decided to use it to join together more powerful components like polyacrylamide. This approach makes the overall material to last for a long time and yet enables it to weaken or disintegrate upon exposure to an optimal wavelength of light.
Raman also built the material as a “double network,” where a single polymer network encloses another polymer network.
You’re forming one polymer network and then forming another polymer network around it, so it’s really entangled. That makes it very tough and stretchy.
Ritu Raman, Study Lead Author and Postdoc, Koch Institute for Integrative Cancer Research, MIT
By changing the composition of the gel, the properties of the material can be altered. When the light-responsive linker constitutes a higher material percentage, it breaks down more quickly in response to light, and at the same time, it is mechanically weaker.
Moreover, by using different wavelengths of light, the scientists can regulate the duration it takes to break down the material. Although blue light works more gradually, it does not pose a major risk to cells that are susceptible to damage caused by ultraviolet light.
Deflated by Light
The gel and its disintegrated products are biocompatible, and it is possible to mold the gel into many different shapes. In the latest analysis, the scientists utilized the gel to reveal two potential uses—an esophageal stent and a seal for a bariatric balloon.
Typical bariatric balloons, which are occasionally used for treating obesity, are first inflated in the stomach of a patient and then filled with saline. Following approximately six months, the bariatric balloon is removed through an endoscopic procedure.
On the other hand, the bariatric balloon developed by the MIT researchers can be deflated by subjecting the seal to a very small LED light, which can be theoretically swallowed and subsequently eliminated from the body.
The researchers’ balloon is composed of latex and also filled with water-absorbing sodium polyacrylate. In the latest research, the scientists tested the bariatric balloons in pigs and discovered that these balloons inflated once they were positioned in the stomach.
However, the bariatric balloons gradually deflated when a tiny, ingestible LED-producing blue light was positioned in the stomach for approximately six hours. In the presence of a higher-power light, the material disintegrated within a period of 30 minutes.
Furthermore, the investigators molded the light-responsive gel into an esophageal stent. Stents like these are occasionally used for treating various disorders, including esophageal cancer, responsible for narrowing the esophagus.
When a light-triggerable version is no longer required, it can be broken down and allowed to travel via the digestive tract.
Apart from a couple of applications, the new method can also be used for developing other types of degradable devices, like vehicles for transmitting drugs to the GI tract, stated the scientists.
“This study is a proof of concept that we can create this kind of material, and now we’re thinking about what are the best applications for it,” stated Traverso.
The study was financed by the Koch Institute Support (core) Grant from the National Cancer Institute, the Bill & Melinda Gates Foundation, the National Institutes of Health, and an AAAS L’Oréal USA for Women in Science Fellowship.