Nov 29 2019
Organic LED displays have become popular among a growing number of electronics manufacturers, for use in computers, televisions, and smartphones. This is because these displays are not only brighter but also provide a greater range of colors.
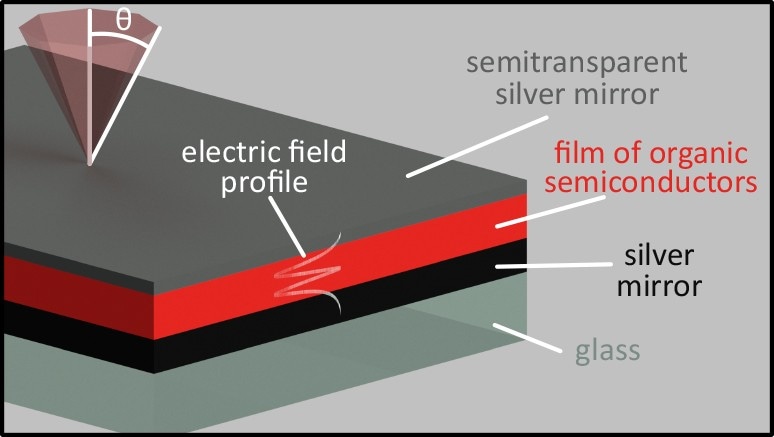 Researchers created a sandwich structure of mirrors made of silver to trap light and force it to interact with a layer of molecules, enhancing the brightness of the molecules’ “dark states.” Image Credit: Cornell University.
Researchers created a sandwich structure of mirrors made of silver to trap light and force it to interact with a layer of molecules, enhancing the brightness of the molecules’ “dark states.” Image Credit: Cornell University.
These devices are powered by organic semiconductors that are extremely flexible and can be easily regulated. In addition, organic semiconductors can be produced in large quantities and more readily when compared to inorganic semiconductors like silicon. Inorganic semiconductors generally need more elevated temperatures for processing.
However, purely organic LEDs have one major drawback—they lose up to 75% of their energy, which makes them extremely wasteful. The reason is organic semiconductors tend to enter “dark states” that prevent them from emitting light. At times, these dark states can even cause the devices to break down. Scientists have been seeking ways to harness the dark states or even jettison them collectively.
Now, in a collaborative study, researchers have identified a new method to prevent the organic semiconductors from going dark. The study was headed by Andrew Musser, assistant professor of chemistry and chemical biology in the College of Arts and Sciences, and also Jenny Clark from the University of Sheffield, United Kingdom.
Microcavities are very small sandwich structures of mirrors. Musser used these microcavities to capture light and force it to communicate with a molecular layer, thus creating a novel hybrid state referred to as a polariton. This hybrid state mixes matter and light. The method may result in brighter and more efficient solar cells, sensors, and LEDs.
The researchers’ article titled “Manipulating Molecules with Strong Coupling: Harvesting Triplet Excitons in Organic Exciton Microcavities” has been published in Chemical Science on November 27th, 2019.
In the LED world, people are putting huge efforts into designing these vast libraries of molecules and testing them in different device configurations to see if, by tweaking the bonds or changing energy levels, they can harvest these dark states more efficiently. It’s a cumbersome, difficult battle because it’s really hard to design molecules. And you don’t necessarily know how to make them do what you want.
Andrew Musser, Assistant Professor, Department of Chemistry and Chemical Biology, The College of Arts and Sciences, Cornell University
“So what we’ve done here is address that problem with a standard molecule, purely by putting it between these mirrors and tuning the way it interacts with light,” Musser added. “This suggests that, for some phenomena, we can bypass a lot of this cumbersome synthetic exploration and tune the molecules at a distance.”
Musser began to focus on polaritons while he was exploring ways to enhance the light-harvesting efficiency in solar cells using organic semiconductors. In such a case, molecules experience a process known as singlet fission. In this process, molecules absorb a single photon and divide that energy into a couple of “packets”—basically two excited electrons—–thus increasing the photon current efficiency in the solar cell by two-fold.
Musser started to investigate how this reverse process takes place, with two packets of energy integrating into one, high-energy state. This state can discharge a high-energy photon. That eventually led Musser to microcavities and the ways these basic optical structures can have a major impact on organic material via light.
Apart from exploiting the electronic characteristics of a molecule for improved brightness, a new study has revealed that these optical structures can also be utilized to target certain bonds and alter their chemical reactivity.
According to Musser, different molecules interact differently with light in the microcavities, and more studies are required to investigate the rules that govern their behavior.
“Right now, it serves to show that when you have these complex materials and you do something even more complicated with the—putting them between these mirrors—weird and wonderful things can happen,” stated Musser.
This work literally sheds light on dark states. We’ve shown that we can use polaritons to force dark states to emit light. Apart from immediate applications for LEDs, this offers a new method for studying organic semiconductors more broadly, using previously unavailable techniques.
Jenny Clark, University of Sheffield
Study co-authors include scientists from the University of California, San Diego, the University of Sheffield, the University of Kentucky, and the University of Cambridge.
The study was supported by the Engineering and Physical Sciences Research Council in the United Kingdom, the University of Sheffield, the European Commission, the European Graphene Flagship Project, and the U.S. Department of Energy. It was performed at the Lord Porter Laser Facility at the University of Sheffield.