Jul 25 2019
In a big step toward creating portable scanners that can quickly measure molecules in pharmaceuticals or categorize tissue in patients’ skin, scientists have developed an imaging system that uses lasers adequately small and efficient to be placed on a microchip.
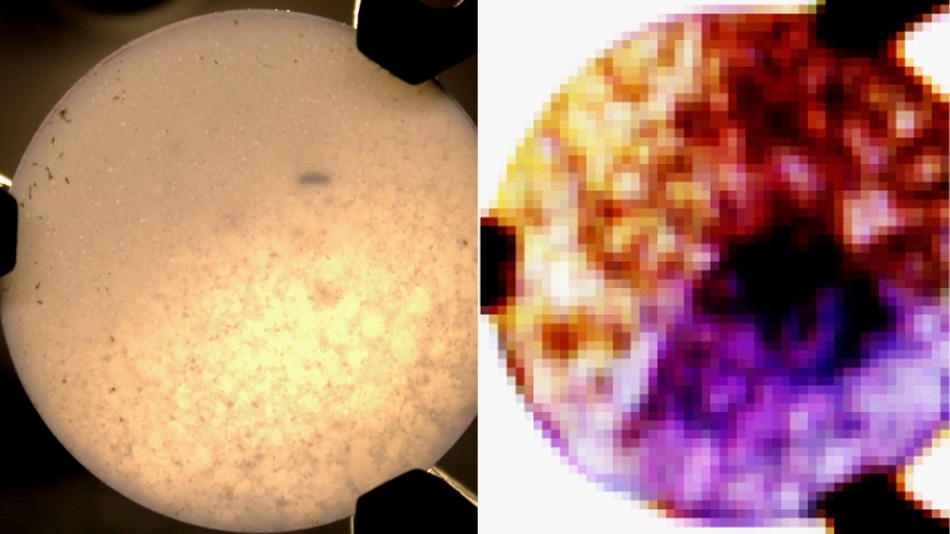 A new imaging technology rapidly measures the chemical compositions of solids. A conventional image of a sample pill is shown at left; at right, looking at the same surface with terahertz frequencies reveals various ingredients as different colors. Such images would aid quality control and development in pharmaceutical manufacturing, as well as medical diagnosis and treatment. (Images courtesy of the researchers.)
A new imaging technology rapidly measures the chemical compositions of solids. A conventional image of a sample pill is shown at left; at right, looking at the same surface with terahertz frequencies reveals various ingredients as different colors. Such images would aid quality control and development in pharmaceutical manufacturing, as well as medical diagnosis and treatment. (Images courtesy of the researchers.)
The system releases and detects electromagnetic radiation at terahertz frequencies—higher than radio waves but lower than the long-wave infrared light applied for thermal imaging. Imaging using terahertz radiation has long been a goal for engineers, but the challenge of developing practical systems that function in this frequency range has thwarted most applications and resulted in what engineers refer to as the “terahertz gap.”
Here, we have a revolutionary technology that doesn’t have any moving parts and uses direct emission of terahertz radiation from semiconductor chips.
Gerard Wysocki, Study Co-Lead and Associate Professor of Electrical Engineering, Princeton University
Terahertz radiation can pierce substances such as plastics and fabrics, is non-ionizing and thus, safe for medical applications, and can be used to observe materials hard to image at other frequencies. The new system, illustrated in a paper published in the June issue of the journal Optica, can swiftly probe the identity and set up of molecules or reveal structural damage of materials.
The device uses steady beams of radiation at specific frequencies. The arrangement is called a frequency comb as it contains numerous “teeth” that each release a diverse, well-defined frequency of radiation. The radiation relates to molecules in the sample material. A dual-comb structure enables the instrument to efficiently calculate the reflected radiation. Distinct patterns, or spectral signatures, in the reflected radiation allow scientists to identify the molecular arrangement of the sample.
While current terahertz imaging technologies are costly to develop and inconvenient to work, the new system is based on a semiconductor design that costs less and can produce numerous images per second. This speed could make it valuable for real-time quality control of pharmaceutical tablets on a production line and other applications that are very fast-paced.
Imagine that every 100 microseconds a tablet is passing by, and you can check if it has a consistent structure and there’s enough of every ingredient you expect.
Gerard Wysocki, Study Co-Lead and Associate Professor of Electrical Engineering, Princeton University
As a proof of concept, the scientists developed a tablet having three zones holding typical inert ingredients in pharmaceuticals—forms of lactose, glucose, and histidine. The terahertz imaging system recognized each ingredient and exposed the boundaries between them, as well as a few areas where one chemical had spilled over into another area. This type of “hot spot” signifies a frequent issue in pharmaceutical production that happens when the active ingredient is not appropriately mixed into a tablet.
The team also showed the system’s resolution by using it to image a U.S. quarter. Minute details like an eagle’s wing feathers, as small as one-fifth of a millimeter wide, were plainly visible.
While the technology makes the medical and industrial use of terahertz imaging more viable than before, it still needs to be cooled to a low temperature, a huge obstacle for practical applications. A number of scientists are currently working on lasers that will possibly work at room temperature. The Princeton team said its dual-comb hyperspectral imaging method will function well with these new room-temperature laser sources, which could then pave the way for more uses.
Since it is non-ionizing, terahertz radiation is harmless for patients and could possibly be used as a diagnostic tool for skin cancer. Furthermore, the technology’s ability to image metal could be used to check airplane wings for damage after being hit by an object while flying.
Besides Wysocki, the paper’s Princeton authors are former visiting graduate student Lukasz Sterczewski (presently a postdoctoral scholar at NASA’s Jet Propulsion Laboratory) and associate research scholar Jonas Westberg. Other co-authors are Yang Yang, David Burghoff, and Qing Hu of the Massachusetts Institute of Technology; and John Reno of Sandia National Laboratories. Support for the study was provided partly by the Defense Advanced Research Projects Agency and the U.S. Department of Energy.