Jan 23 2019
Chemical sensors, drug delivery, and fluid pumps could now be improved with an innovative, simple, and low-cost technique that utilizes ultraviolet light to regulate the motion and assembly of particles inside liquids.
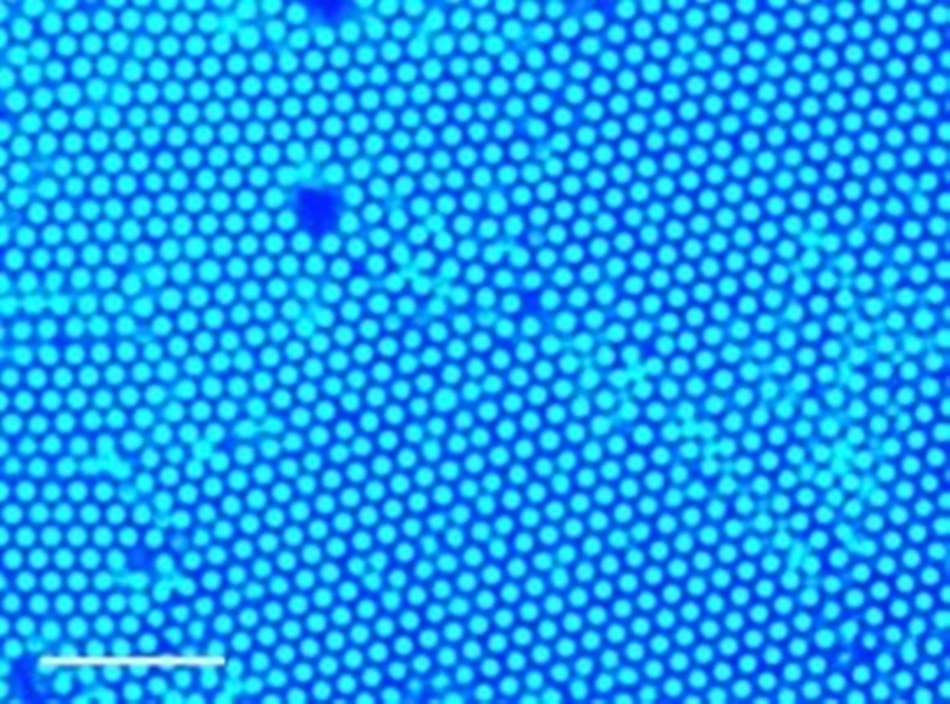 A new method uses ultraviolet light and small amounts of gold or titanium dioxide nanoparticles to gather larger particles at the point of light. This method was used to gather polystyrene particles, which form a well-packed structure called a colloid crystal, as depicted in this image. (Image credit: Sen Lab, Penn State)
A new method uses ultraviolet light and small amounts of gold or titanium dioxide nanoparticles to gather larger particles at the point of light. This method was used to gather polystyrene particles, which form a well-packed structure called a colloid crystal, as depicted in this image. (Image credit: Sen Lab, Penn State)
In the latest technique, particles—ranging from pollutants to bacterial spores and plastic microbeads—are encouraged to collect and organize at a particular location inside a liquid and, if required, to shift to new locations. A paper detailing this new approach has been published in the journal Angewandte Chemie.
Many applications related to sensors, drug delivery, and nanotechnology require the precise control of the flow of fluids. Researchers have developed a number of strategies to do so, including nanomotors and fluid pumps, but prior to this study we did not have an easy way to gather particles at a particular location so that they can perform a useful function and then move them to a new location so they can perform the function again. Say for example you want to build a sensor to detect particles of a pollutant, or bacterial spores in a water sample. With this new method, we can simply add nanoparticles of gold or titanium dioxide and shine a light to encourage the pollutant particles or spores to gather. By concentrating them in one spot, they become easier to detect. And because light is so easy to manipulate, we have a high degree of control.
Ayusman Sen, Study Senior Author and Distinguished Professor, Department of Chemistry, Penn State.
Just like how pollutant particles can be collected at a specific place, the new technique could be used for collecting polymer or silica beads that are capable of carrying a payload, like drugs or antibodies, at specific sites inside a fluid.
In the novel method, a small quantity of gold or titanium dioxide nanoparticles is initially added to a liquid such as water, in which larger particles of interest like beads or pollutants carrying a payload are also present. When a particular point in the liquid is illuminated with light, the minute metal nanoparticles get heated up and the heat is subsequently transferred to the fluid. Similar to how warm air increases in a chilly room, the warmer liquid subsequently rises at the point of light, while cooler water rushes in to fill the space left by the warm water and brings the larger particles with it.
This causes the larger particles to collect at the point of UV light, where they form closely packed, well-organized structures called colloidal crystals. Changing the intensity of the light or the amount of titanium dioxide or gold particles alters how quickly this process occurs.
Benjamin Tansi, Study First Author and Graduate Student, Department of Chemistry, Penn State.
Upon removing the light, the larger particles arbitrarily diffuse via the liquid; however, relocating the light causes the larger particles to shift toward the new point of light and allows them to considerably retain their structure as they move. This dynamic movement, assembly, and disassembly of organized particles may prove useful for drug delivery and sensing applications.
“This process is most efficient when gold nanoparticles are used, but we wanted to find an alternative that was less expensive and more accessible,” stated Tansi. “We were pleased to find that this method also works with titanium dioxide, an inexpensive and harmless nanoparticle used in cosmetics and as a food additive.”
Besides water, the scientists showed the effectiveness of this technique in an organic liquid, hexadecane.
Particles usually don’t assemble very well in salty or non-aqueous environments because everything sticks together. But here we show that particles can assemble using this method in hexadecane, which suggests we may be able to apply this technique in, for example, biological fluids. To our knowledge this is the first demonstration of light-driven fluid pumping in an organic medium.
Ayusman Sen, Study Senior Author and Distinguished Professor, Department of Chemistry, Penn State.
Mathematical models were used by research team members at the University of Pittsburgh headed by Anna Balazs to elucidate the system’s dynamics. Apart from detailing the way particles move in the system, the models also showed that only a slight temperature change—that is, less than a degree Celsius—from the ultraviolet light is needed to promote the fluid flow.
At present, the researchers are testing the limitations of this technique, for instance, whether the method can be used for sorting particles by size, or whether particles can move uphill toward the light source.
“We knew that heating gold nanoparticles in suspension could create a fluid flow,” said Tansi, “but prior to this study no one had looked to see if these kinds of thermally-driven fluid flows could be used to do anything useful. Because ultraviolet light and titanium dioxide are so easy to control, we think this method could be harnessed in various technologies in the future. For example, a fluid pump that relies on this method could potentially replace the bulky and more expensive traditional pumps that require a power source or that rely on magnetics or mechanical movement to function.”
Apart from Sen, Balazs, and Tansi, the research team includes Oleg Shklyaev from the University of Pittsburgh and Matthew Peris from Penn State. The National Science Foundation funded the study.
New method to control fluid flow
Using the new method, the researchers gather the particles of interest into an organized structure at the point of light (left). When the light is moved to a new location, the particles move toward the new point of light (right), as depicted in this video. (Video credit: Sen Lab, Penn State)