Dec 12 2015
A laser treatment called Novilase® Breast Therapy shows promise as a way to successfully treat small breast cancers, according to research that was led by principal investigator Barbara Schwartzberg, M.D. of Rose Medical Center.
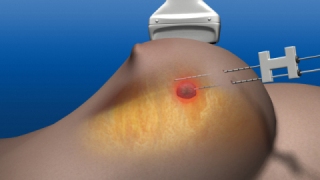 Laser treatment of early breast cancer could replace lumpectomy in the future. A new study found that Novilase Breast Therapy, a minimally invasive procedure, holds promise for patients. (Photo: Business Wire)
Laser treatment of early breast cancer could replace lumpectomy in the future. A new study found that Novilase Breast Therapy, a minimally invasive procedure, holds promise for patients. (Photo: Business Wire)
Results of the multi-center international BR-002 Novilase Clinical Trial were presented today by Dr. Schwartzberg in a scientific poster at the prestigious 2015 San Antonio Breast Cancer Symposium (SABCS).
Laser ablation treatment places small probes in the center of the cancer and subsequently uses heat to destroy tumors. Laser ablation could be a viable option for small lesions and might allow some women to forgo surgery in the future.
The trial results showed that Novilase Breast Therapy achieved 91 percent complete tumor ablation when treatment guidelines were followed. The trial also demonstrated that MRI can be used to determine effectiveness of tumor ablation. As such, MR imaging may potentially be used to assess effectiveness, as pathology is used today after surgery.
The research trial, headed by Dr. Schwartzberg, is part of the process of determining whether Novilase Breast Therapy will be approved by the U.S. Food & Drug Administration (FDA) as an effective alternative to lumpectomy surgery for the treatment of small, early-stage breast cancers.
All patients in the study received standard-of-care, adjuvant and radiation therapy as indicated. The ablated tissue was excised with surgery 4 weeks after the laser treatment to allow investigators to analyze the pathology of the targeted lesion. Researchers used that data to correlate imaging to pathology in evaluating disease status after the procedure.
The Novilase laser treatment, developed by Novian Health, is a minimally invasive alternative to lumpectomy that targets the lesion using ultrasound guidance. The trial results point to a possible future in which women could get common breast cancers treated with less physical damage and disruption to their daily lives. The novel Novilase laser ablation therapy is already FDA-cleared as an alternative to surgery for the treatment of benign breast tumors (fibroadenomas).
“We learned more from this research study than we anticipated, so I’m very pleased with the results,” said Dr. Schwartzberg. “We saw multiple advantages of using laser therapy to not only destroy the cancer tumor, but to do so with only local anesthetic and less cosmetic damage than traditional lumpectomy. Patients benefit from quicker recovery and are less likely to need additional treatments than with surgery.”
Even when retreatment is needed, the consequences are far less with the laser than with a lumpectomy, Dr. Schwartzberg said: “A retreatment with the laser is still a minimally invasive procedure that causes no substantial change to the look and feel of the breast and otherwise poses few risks for the patient. By comparison when a second surgery is needed, the situation is completely different. You’re probably worsening the cosmetic impact already caused by the first operation. Plus you’re re-exposing patients to the other risks and stresses of surgery.”
The clinical trial, which included Rose Medical Center and its breast program, was conducted at 11 different sites – eight in the U.S. and three in Britain – with both radiologists and breast surgeons as investigators. A total of 61 patients with 64 tumors (61 primary and three satellite) were enrolled in the study. Notable results included:
- High rate of successful treatment. In 91 percent of cases where Novilase Breast Therapy was performed according to technical guidelines, the tumor was completely ablated. In all cases, there was 84 percent complete tumor ablation.
- Positive health-related quality of life scores. Patients reported better symptom-related scores and higher functional status scores following Novilase than has been reported for surgery.
- No serious adverse events. There were no serious adverse events related to Novilase therapy at any of the trial sites.
- Correlation between MRI and pathology. The trial demonstrated a high rate of correlation between MRI and pathology in determining effectiveness of laser ablation of breast cancer (a negative predictive value of 92 percent in correlating with pathology). In other words, the research showed that an MRI may be effective means of determining if a patient’s treatment was successful.
“The radiology aspects of the trial were very encouraging,” said Rose Medical Center radiologist John Lewin, M.D., a co-author of the poster presented at the San Antonio conference. “When targeted appropriately using the image-guidance technology, the laser treatment achieved complete ablation of the tumor a very large percentage of the time. The tight correlation between MRI and pathology was also impressive. Using imaging instead of pathology to analyze treatment outcomes is almost as pioneering and important as using a laser instead of surgery.”
If the trial results help lead Novilase Breast Therapy to becoming approved as a breast cancer treatment option, women could benefit in several ways. Rather than requiring a surgical incision, the laser device is inserted through the skin into the tumor. The incisions are so small that they are closed with a bandage after the procedure. Laser treatment causes little or no scarring or change in the breast’s shape and feel – in contrast to the substantial cosmetic and tactile impact that can result from a lumpectomy.
Laser treatment requires only local anesthesia instead of the general anesthesia or IV sedation used for breast cancer lumpectomy or surgery. Laser patients can generally return to normal activities in a day or less, while recovery from surgery may take up to a week. Finally, there is also less risk of complications with Novilase Breast Therapy.
“The difference between the laser and my two lumpectomies was day and night,” said Kathryn Lawrence, 51, a graphic designer and property investor in Brighton, Colo. She participated in the clinical trial at Rose Medical Center and has had lumpectomies for cancers in both of her breasts.
“The laser was almost pain-free with no scars and no deformity. I was able to attend a business meeting on the same day as my treatment,” she said. “I hope my participation in the trial helps to make this option available to women like me in the future. I was excited to be one of the first to access this technology for my diagnosis of breast cancer and look forward to the day when any one who is diagnosed can take advantage of this incredible treatment option.”
A follow-up pivotal clinical trial of the Novilase technology is planned for 2016. It will expand upon the findings from the recent BR-002 study, as part of the process to make the laser treatment more widely available for women with breast cancer.