Feb 14 2013
Unravelling electron and energy transfer processes of amino-acid residues in bio-systems
 © 2013 EPFL
© 2013 EPFL
The past twenty years have witnessed a significant effort aimed at pushing the methods of multidimensional NMR spectroscopies from the radiofrequency domain into the optical domain, first in the infrared (vibrational multidimensional spectroscopy) and around the mid-2000’s in the visible range (electronic multidimensional spectroscopy), with the advantage of probing couplings between vibrational or electronic dipoles (chromophores) in biological systems that are orders of magnitude stronger than in NMR, while achieving a much higher time resolution of sub-picoseconds.
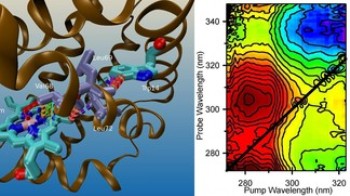
Pushing 2D spectroscopy further into the UV below 300 nm brings the additional advantage of accessing the absorption region of amino acid residues and nucleic acids. However, because of their large (30-50 nm) absorption band widths, 2D UV spectroscopy has remained a challenge. Recently, the group of Prof. Chergui has implemented the first experimental set-up for 2D UV spectroscopy and in a recent article in Science, they demonstrated its capabilities in the case of heme proteins. Indeed, in addition to disentangling the broad overlapping spectral contributions of three chromophores (the heme and the two tryptophan residues), they identified electron-transfer processes between one of the tryptophans and the heme. So far, it was always assumed that the tryptophan fluorescence is quenched by an energy transfer (so-called FRET) to the heme. These results question the systematic use of FRET to interpret the decay of the Trp fluorescence in biological systems, and therefore its general role as a “spectroscopic ruler”. On a broader perspective, the ability of 2D UV spectroscopy to detect couplings between chromophores in biosystems, offers a new tool to study protein-target interactions.