Advanced atomic force microscopy (AFM) techniques can be used to capture real-time images of molecules changing their charge state.

Image Credit: lucadp/Shutterstock.com
Charging and discharging of molecules are essential mechanisms in converting and transporting energy in living organisms, including the charge states of chlorophyll and hemoglobin. Understanding charge state conditions of molecules is also crucial for developing photovoltaic devices.
The structure and function of a molecule change when charged.
To better understand the foundations of molecular structure, high-resolution images of the charging process are appropriate.
AFM is an effective tool that enables atomic-scale imaging of materials and surfaces.
Recent advances in AFM technology have enabled the imaging of molecule structure changes when its charge state is altered by adding or removing a single elementary charge (one electron).
Working Principle of AFM
The significant elements of an AFM setup are shown in figure 1 (a).
A sharp metallic tip is used to scan a sample's surface. The AFM tip is usually silicon or silicon nitride and is attached at one end of a flexible cantilever.
As the AFM tip scans over different sample surface features, the cantilever compresses and stretches like a spring.
A laser beam directed to the back of the cantilever is deflected by the oscillations of the AFM tip. A position-sensitive photodetector records the small variations of the sample surface topography.
The AFM can be operated in several modes depending on the experiment's goals. The operational modes of the AFM are categorized as contact mode and non-contact mode. In contact mode, the AFM tip drags across the sample surface. The tip is made to oscillate near the sample's surface without contact in the non-contact mode.
Qplus AFM
AFM tips used as force sensors with optical detection are mainly machined from silicon. An innovative variation of the AFM tip, the QPlus sensor, was introduced by German physicist Franz Josef Giessibl.
In the Qplus probe, the silicon tip is replaced by an electrically active quartz tip (see figure 1 (b)).
Quartz has a piezoelectric property that enables self-sensing. The piezoelectric effect helps generate an electric charge in response to applied mechanical stress. This capability can replace using a laser to detect the AFM tip movements.
Qplus probes are usually operated in non-contact mode. An applied frequency modulation to the AFM tip oscillates the cantilever. Small alterations sensed by the tip due to force variations felt from the sample surface are detected.
Qplus AFM method is capable of unprecedented subatomic spatial resolution, force detection with sub-piconewton sensitivity and imaging and spectroscopy of spin-dependent forces.
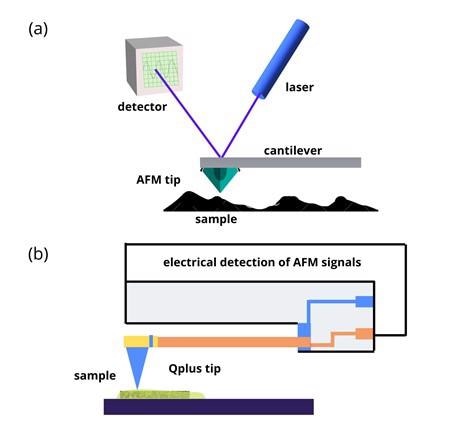
Figure 1: (a) Schematic illustrations showing basic principles of AFM. (b) AFM with Qplus probe. Image Credit: Ilamaran Sivarajah
Functionalized Tips
Attaching specific molecules to an AFM tip converts it into a suitable sensor for molecular imaging.
Chemically adhering agents to improve tip sensitivity is referred to as tip functionalizing. Carbon monoxide (CO) functionalized tips display remarkable enhancement in the resolution of covalent bonds in molecules.
Examples of Molecular Charge States Imaged by AFM
In recent experiments where AFM was used to image molecular charge states, azobenzene, pentacene, tetracyanoquinodimethane (TCNQ) and porphine were studied.
The samples were held in a vacuum chamber to shield them from environmental influences in the experimental setup. A small voltage bias introduced to the Qplus probe allowed the transfer of individual electrons to the molecule.
The experiments on all four molecules were carried out using the following control conditions:
- Imaging the molecule in its neutral state without adding or removing an electron
- Positively charging the molecule by removing an electron
- Adding an electron to charge the molecule negatively
- Adding two electrons to make the molecule double negative
Each molecule produced a uniquely different AFM image for all other experimental conditions.
The pentacene molecule became more reactive by increasing additional electrons.
The azobenzene molecule returned a physically twisted image.
The bonds between the atoms that constitute the TCNQ molecule moved and changed during charging. The atomic bonds stretched and changed sizes in porphine during the charging process.
Outlook
Imaging molecular processes of charge states significantly impact molecular energy transfer understanding. Specific fundamental methods in biology such as chlorophyll and hemoglobin are made up of molecules like porphine.
Resolving the charging process of such molecules can support strategic resource implementation and drug discoveries.
References and Further Reading
Franz J. Giessibl. (2019) The qPlus sensor, a powerful core for the atomic force microscope. Review of Scientific Instruments. 90, 011101. https://doi.org/10.1063/1.5052264
Pürckhauer, K., Weymouth, A.J., Pfeffer, K. et al. (2018) Imaging in Biologically-Relevant Environments with AFM Using Stiff qPlus Sensors. Sci Rep 8, 9330. https://doi.org/10.1038/s41598-018-27608-6
Jiayu Xu, Xiang Zhu, Shijing Tan, Yao Zhang, Bin Li, Yunzhe Tian, Huan Shan, Xuefeng Cui, Aidi Zhao, Zhenchao Dong, Jinlong Yang, Yi Luo, Bing Wang, J. G. Hou. (2021) Determining structural and chemical heterogeneities of surface species at the single-bond limit, Science, 371, 6531, (818-822) https://doi.org/10.1126/science.abd1827
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.