For the first time, scientists have achieved the selective excitation of a molecule by employing a combination of two extreme-ultraviolet light sources, leading to the molecule’s controlled dissociation while meticulously tracking its evolution over time.
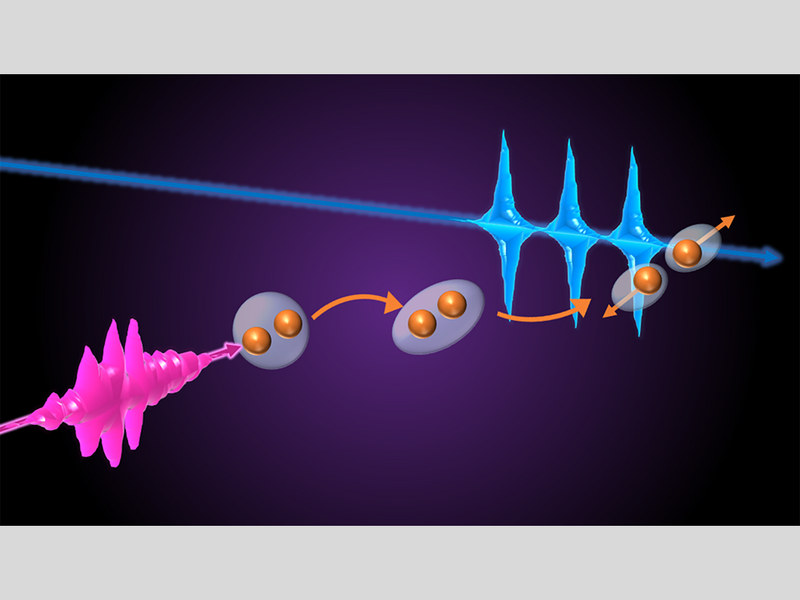 An XUV laser pulse (pink) excites an oxygen molecule (orange). The molecule dissociates into different atomic fragments, which can be “photographed” by another XUV laser pulse (blue). Image Credit: Max Planck Institute for Nuclear Physics.
An XUV laser pulse (pink) excites an oxygen molecule (orange). The molecule dissociates into different atomic fragments, which can be “photographed” by another XUV laser pulse (blue). Image Credit: Max Planck Institute for Nuclear Physics.
This milestone marks a significant stride toward the precise quantum mechanical manipulation of chemical reactions, opening up potential avenues for novel and previously undiscovered reaction pathways.
The interplay between light and matter, particularly with molecules, holds pivotal significance in various natural phenomena, including biological processes like photosynthesis. This phenomenon is also harnessed in technologies such as solar cells, predominantly utilizing visible, ultraviolet, or infrared light on Earth’s surface.
Notably, extreme-ultraviolet (XUV) light, possessing substantially higher energy than visible light, is absorbed by the atmosphere and does not reach the Earth’s surface naturally. However, XUV radiation can be artificially generated in the laboratory, offering a means to selectively excite electrons in molecules.
In a molecule, while the outermost electrons function as a kind of "chemical glue," binding individual atoms together, inner shell electrons are closer to the atomic nucleus and exhibit greater localization within the molecule.
It is precisely these inner shell electrons that can now be selectively excited using XUV radiation, paving the way for unprecedented chemical reaction processes not occurring naturally on Earth’s surface.
A collaborative effort led by PD Dr. Christian Ott’s group in the Department of Prof. Pfeifer at the Max-Planck-Institut für Kernphysik in Heidelberg, Germany, has achieved a breakthrough by successfully integrating two distinct XUV light sources. This integration allowed the temporal resolution of a quantum mechanical dissociation mechanism in oxygen molecules.
To achieve this feat, laser pulses are generated through the process of high harmonic generation (HHG), wherein infrared light is channeled through a gas cell, transforming it into XUV radiation—a technique notably acknowledged in this year's Nobel Prize in Physics.
Additionally, a free-electron laser (FEL) is employed, wherein accelerated electrons emit XUV light. Both methodologies produce XUV pulses with a duration as brief as femtoseconds, a millionth of a billionth of a second.
The crucial element lies in the distinctiveness of the spectra of the two laser pulses.
“The HHG pulses have a very broad spectrum, which means they consist of light with many different frequencies – in the visible range this could be understood as different colors. The FEL pulses, on the other hand, are much more limited spectrally,” explains PhD Student and First Author of the Study Alexander Magunia.
The free-electron laser (FEL) pulses, generated at the FLASH@DESY free-electron laser in Hamburg, are utilized to selectively excite the electrons of the oxygen molecule, placing them in a specific state.
It is well-established that this state triggers the molecule’s dissociation through two distinct channels. However, the speed of this process has remained uncertain until now.
The challenge arises from the fact that the atoms in the oxygen molecule undergo a “quantum tunneling” process, introducing complexities in providing precise theoretical descriptions. To address this, a second high harmonic generation (HHG) pulse, with an adjustable time delay, is introduced alongside the initial stimulating FEL pulse.
This experimental setup enables the observation of molecular dissociation, akin to capturing frames in a rapid photo series.
The HHG pulses play a crucial role by allowing the simultaneous “photographing” of all resulting fragments through their spectral absorption fingerprints. The variation in time delay between the two pulses directly correlates with the number of molecules that have undergone decay. This increase in fragments provides the means to determine the duration of the process and the respective rates for the two decay channels.
The ability to initiate targeted electronic or molecular processes with FEL pulses, coupled with the independent retrieval of extensive quantum-mechanical state information through the broadband HHG spectra, holds promise for recording, comprehending, and potentially controlling more intricate chemical reactions using light in the future.
Journal Reference:
Mangunia, A., et al. (2023) Time-resolving state-specific molecular dissociation with XUV broadband absorption spectroscopy. Science Advances. doi.org/10.1126/sciadv.adk1482.