An international research team led by KU Leuven researcher Ventsislav Valev has developed a new method for controlling light at the nanoscale level to achieve optical detection of single molecules.
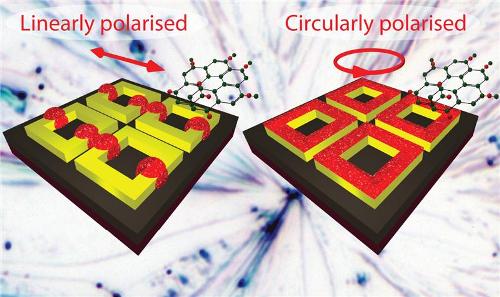 Shining circularly polarised light on ring-shaped nanostructures increases the opportunity for interaction with molecules
Shining circularly polarised light on ring-shaped nanostructures increases the opportunity for interaction with molecules
The researchers activated the overall surface of the nanostructure by illuminating the gold, square-ring shaped nanostructure with circularly polarised light, making it feasible for molecular interaction. The method can be used for visually observing single molecules as well as multiple-molecule interactions.
Since single molecules have weak optical responses, several global researchers of nanotechnology are looking out for a proper method to optically detect single molecules. However, scientists have developed a method where light can be focused on tiny spots known as ‘hotspots’ using metal nanostructures. After causing the electrons to excite on the surface of the nanostructure, hotspots will oscillate them coherently. Based on these oscillating electrons, the focus of light on the electrons will enhance the optical signal of the molecule to 100 billion times its normal strength. An optical microscope will allow the detection of this signal.
However, this method has two limitations, which include the overheating of hotspots that can melt the nanostructures and the miniature size of the hotspot.
Dr. Valev and his team devised nanoengineering larger spots for overcoming these limitations. A circularly polarised light was used for illuminating nanostructures rather than linearly polarised light. Besides providing a larger area, the circularly polarized light radiated on square-ring shaped gold nanostructures, thereby effectively activating the entire surface of the nanostructure.
This novel method can be deployed for extensive potential applications in nanoscale photochemistry. In addition, it supports technology advancement for visualizing multiple- and single-molecule interactions.
The findings have been published in the scientific journal Advanced Materials.