Powerful X-Ray beams were utilized by a research group headed by the U.S. Department of Energy’s (DOE) Argonne National Laboratory to find new materials crucial to the production and use of hydrogen.
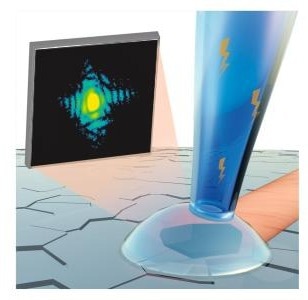
An intense X-ray beam (in pink) is focused into a small spot on a single nanoscale grain of a platinum electrode (highlighted within the droplet). Diffraction interference patterns from that grain were collected on an X-ray detector (the black screen). (Image Credit by Dina Sheyfer, Argonne National Laboratory)
The aim is to make the production of hydrogen and usage highly efficient and affordable, thereby providing better fuel for transportation and industry.
Efficient hydrogen production is key. Hydrogen is the lightest energy storage material. Hydrogen can be produced from water using renewable energy or excess energy, transported as a fuel, and converted back to water to produce energy for consumers. Platinum and its alloys are best in catalyzing and boosting the water-splitting process by accelerating the exchange of electrons.
Hoydoo You, Senior Physicist, Argonne National Laboratory
Understanding and developing materials, allowing effective production and use of hydrogen, are integral to the hydrogen economy. The initial step was made by scientists in coming up with a tool that allows them to specify the materials with a new level of detail, eventually making the best materials for hydrogen production and use.
You stated, “This will make production and use of hydrogen less costly and more environmentally friendly.”
The research group utilized the Advanced Photon Source (APS), a DOE Office of Science user facility at Argonne. Having been working at the APS, scientists aimed an intense X-Ray beam onto a single grain of platinum. Diffraction patterns from that grain were gathered on an X-Ray detector. Those patterns were then shifted into images of the sample with the help of tailored computer algorithms.
A nanodroplet chemical cell, made with a small pipette tip (a tool for creating a small droplet of liquid), was utilized to regulate the chemical reaction occurring on the platinum grain to produce hydrogen in an electrolyzer. An electrolyzer is known as a device for producing hydrogen fuel from water utilizing electricity; the device in a reverse operation, called a fuel cell, shifts hydrogen fuel back to electricity.
“The reaction was controlled by applying voltage, directed through an electrolyte in the nano-pipette onto the grain being studied,” stated Argonne physicist Matt Highland. He designed the initial prototype of this new tool.
This prototype allowed the analysis of a single nanograin and set the stage for scanning capability over all grains in a realistic electrolyzer or fuel cell when the APS upgrade has been done.
Ross Harder and Wonsuk Cha are Argonne physicists who worked at the APS beamline 34-ID-C, where the experiments were performed and helped with combining the new electrochemistry tool in the present instrument.
The ability to do localized electrochemistry while creating a new picture of the way things were happening, at a single particle level, was incredible.
Ross Harder, Physicist, Argonne National Laboratory
At present, the APS provides X-Ray beams that are up to a billion times brighter compared to those utilized by a dentist. However, an extensive upgrade will make the APS highly powerful.
While the upgraded APS goes live in 2024, its X-Ray beams will be up to 500 times brighter compared to what is available at present. This implies that methods like the one utilized in this study will get even better following the upgrade.
The APS upgrade will help us see things happen in real time in the material. Measurement times could become fast enough that we can move from one particle to another, and we could see how they are interacting with the electrochemical environment and each other.
Ross Harder, Physicist, Argonne National Laboratory
“Important processes like battery charging and corrosion require the real-time imaging of grains to understand a full picture of the process. We believe the added brightness of the APS upgrade with our new tool will enable studies we can only dream about today,” stated Argonne assistant physicist Dina Sheyfer.
The co-authors of the study are Sheyfer, Highland, Harder, Cha, and You with Argonne’s Stephan O. Hruszkewycz and former Argonne scientist Tomoya Kawaguchi (Tohoku University). The other contributors include Stanford University’s Ruperto G. Mariano, and Matthew W. Kanan.
This study was financially supported in part by the DOE Office of Basic Energy Sciences, Materials Sciences and Engineering Division, and the Scientific User Facilities Division.