X-Ray laser experiments reveal that intense light deforms the structure of a thermoelectric material in an exceptional manner, revealing a new possibility for regulating material properties.
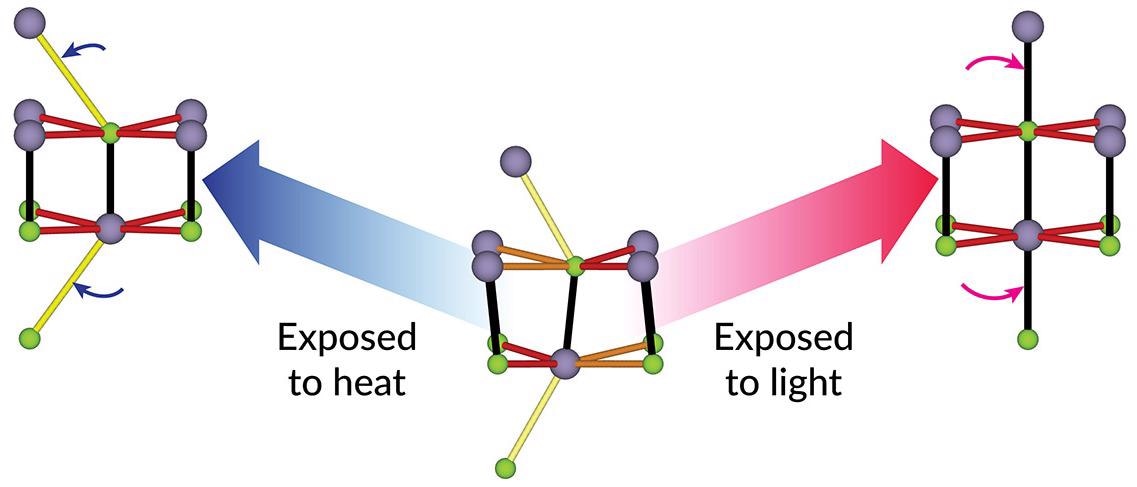 An illustration shows how the atomic structure of tin selenide, a crystalline material that can convert heat to electricity, changes when exposed to heat or ultrafast laser light. The structure in the middle is at room temperature. Heating (left) moves the top and bottom atoms a little further left, from this point of view, and subtly shifts some of the other atoms. Scientists thought exposing the material to ultrafast laser light would do much the same thing; instead, its atoms shifted in new ways (right). SLAC’s X-ray free-electron laser, LCLS, allowed researchers to see these atomic movements and structural distortions for the first time, opening a new avenue to tailoring materials with light. Image Credit: Yijing Huang/Stanford University.
An illustration shows how the atomic structure of tin selenide, a crystalline material that can convert heat to electricity, changes when exposed to heat or ultrafast laser light. The structure in the middle is at room temperature. Heating (left) moves the top and bottom atoms a little further left, from this point of view, and subtly shifts some of the other atoms. Scientists thought exposing the material to ultrafast laser light would do much the same thing; instead, its atoms shifted in new ways (right). SLAC’s X-ray free-electron laser, LCLS, allowed researchers to see these atomic movements and structural distortions for the first time, opening a new avenue to tailoring materials with light. Image Credit: Yijing Huang/Stanford University.
Thermoelectric materials convert heat to electricity and vice versa, and the atomic structures of these materials are closely linked to their performance.
Scientists recently discovered how to use intense pulses of laser light to change the atomic structure of tin selenide, a highly efficient thermoelectric material. The findings pave the way for novel means to enhance thermoelectrics and a host of other materials by manipulating their structure, resulting in materials with dramatic new properties that do not exist in nature.
For this class of materials that’s extremely important, because their functional properties are associated with their structure. By changing the nature of the light you put in, you can tailor the nature of the material you create.
Yijing Huang, Graduate Student, Stanford University
Yijing Huang performed a major role in the experiments at the Department of Energy’s SLAC National Accelerator Laboratory.
The experiments were carried out at SLAC’s X-Ray free-electron laser, the Linac Coherent Light Source (LCLS). The observations were published on February 14th, 2022, in the Physical Review X journal. The findings will also be highlighted in a special collection devoted to ultrafast science.
Heat Versus Light
Thermoelectrics convert waste heat to electricity and hence they are regarded as a form of green energy. Thermoelectric generators provided electricity for the Apollo moon landing project, and since then scientists have been pursuing ways to use thermoelectric generators to convert human body heat into electricity for charging gadgets, among other things.
When reversed, they can produce a heat gradient that could be utilized to chill wine in refrigerators without involving moving parts.
Tin selenide is one of the most promising thermoelectric materials because it is grown as individual crystals and is relatively inexpensive and simple to produce.
According to Huang, tin selenide is a much more efficient heat converter than many other thermoelectric materials because it is lead-free. It is also easy to produce and experiment with as it is made up of regular cube-like crystals similar to those found in rock salt.
To observe how those crystals react to light, the researchers used intense pulses of near-infrared laser light to change the structure of tin selenide. The light excited electrons in the sample’s atoms, causing some of them to shift positions and distort their arrangement.
The scientists then tracked and evaluated those atomic movements and the changes in the structure of the crystals with pulses of X-Ray laser light from LCLS, which rapidly captures changes that occur in just millionths of a billionth of a second.
You need the ultrafast pulses and atomic resolution that LCLS gives us to reconstruct where the atoms are moving. Without that we would have gotten the story wrong.
David Reis, Study Co-Author and Professor, Stanford University
David Reis is also a professor at SLAC and director of the Stanford PULSE Institute.
A Startling Result
The observation was unexpected and when Huang informed her teammates of the observations of the experiments, they had a hard time believing her.
Applying heat is a tried-and-true method of changing the atomic structure of tin selenide. It changes the material in a predictable manner and improves its performance. The traditional wisdom was that using laser light would also help achieve the same effect as heating would.
That’s what we initially thought would happen. But after almost two years of discussion, Yijing finally convinced the rest of the team that no, we were driving the material towards an entirely different structure. I think this result goes against most people’s intuition about what happens when you excite electrons to higher energy levels.
Mariano Trigo, Staff Scientist, SLAC National Accelerator Laboratory
Shan Yang, a graduate student at Duke University, carried out the theoretical calculations and confirmed that this interpretation of the experimental data was the correct one.
“This material and its class are certainly very interesting, because it’s a system where small changes could lead to very different results. But the ability to make entirely new structures with light—structures we don’t know how to make any other way—is presumably more universal than that,” Reis added.
It could be useful in the decades-long quest to produce superconductors — materials that conduct electricity without loss — that operate at close to room temperature, he added.
The study, which was funded by the DOE Office of Science, included researchers from the DOE’s Oak Ridge National Laboratory. The Stanford Synchrotron Radiation Lightsource at SLAC was used to conduct preliminary research (SSRL). SSRL and LCLS are DOE Office of Science user facilities.
Journal Reference:
Huang, Y., et al. (2022) Observation of a Novel Lattice Instability in Ultrafast Photoexcited SnSe. Physical Review X. doi.org/10.1103/PhysRevX.12.011029.