Jul 22 2019
A review on serial femtosecond crystallography has been published by scientists from the Moscow Institute of Physics and Technology (MIPT). Serial femtosecond crystallography is considered as one of the most potent techniques for examining the proteins’ tertiary structure.
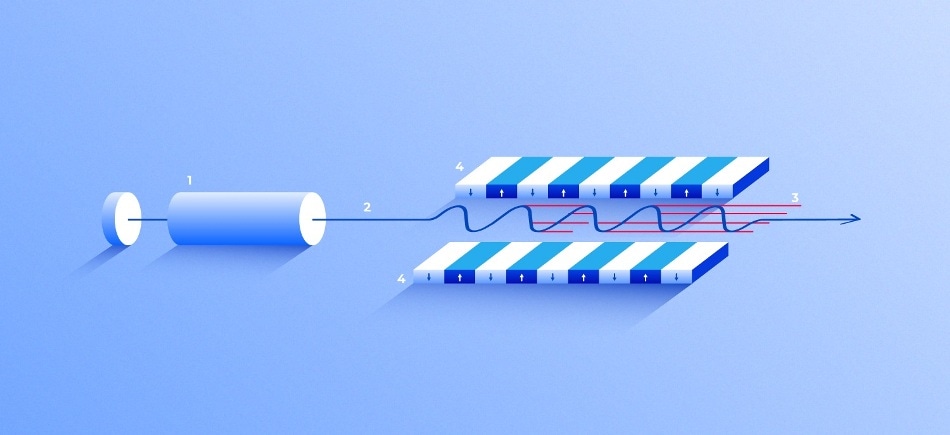 X-ray free-electron laser. A source (1) emits free electrons (2) moving merely tens of times slower than the speed of light through the undulator (4), a tunnel lined with many magnets. The magnetic field causes an electron traveling through the tunnel to oscillate and therefore emit X-rays. The movement of the electrons in the undulator is synchronized to generate tight, high-frequency X-ray pulses of remarkable intensity (3). (Image credit: Elena Khavina/MIPT Press Office)
X-ray free-electron laser. A source (1) emits free electrons (2) moving merely tens of times slower than the speed of light through the undulator (4), a tunnel lined with many magnets. The magnetic field causes an electron traveling through the tunnel to oscillate and therefore emit X-rays. The movement of the electrons in the undulator is synchronized to generate tight, high-frequency X-ray pulses of remarkable intensity (3). (Image credit: Elena Khavina/MIPT Press Office)
This method has advanced quickly over the past 10 years, presenting new opportunities for the sensible design of protein-targeting drugs that earlier were inaccessible to structural analysis. The article has been published in the journal Expert Opinion on Drug Discovery.
X-Ray Crystallography
One of the main techniques for exposing the three-dimensional (3D) structure of biological macromolecules, including proteins, X-ray crystallography has made it possible to establish the structure of various pharmacologically significant receptors and enzymes. This facilitates the design of drugs that target these proteins.
In the X-ray crystallography technique, a protein is crystallized and examined through X-ray diffraction. Initially, the protein is separated and purified, and the solvent dries out slowly. Consequently, the molecules whose structure is being studied create crystals, which are characterized by an internal order.
The researchers successfully achieved a diffraction pattern by exposing a crystal to X-rays in a unique device. This pattern contains data on the positions of atoms within the crystal. A thorough examination of the diffraction pattern exposed the 3D structure of the constituent protein molecules.
Before the introduction of this technique, novel drugs were largely sought empirically—either by sorting through a range of molecules in chemical libraries, or by altering the structure of the molecules that are known to have an impact on the target protein.
Besides, 3D structures of a large number of target proteins are now available that allow scientists to observe them on a PC screen and rapidly sort through a countless number of compounds looking for drug candidates. This also allows them to save a great deal of time and money that were earlier spent on “wet” experiments and chemical synthesis.
X-ray crystallography generates excellent results for crystals that are uniform, stable, and large—that is, they do not have structural defects or impurities. A powerful radiation pulse is required to better identify a weak diffraction signal but it should not be so powerful as to damage the crystal. In traditional X-ray crystallography, diffraction patterns for numerous spatial orientations are produced by rotating a protein crystal in the X-ray beam. This method helps in capturing maximum data on the structure.
Method for Tricky Targets
Soon after the emergence of X-ray crystallography, it became evident that it is not possible to crystallize all biological macromolecules. Certain proteins are normally dissolved in the inner cell medium. Therefore, it is relatively simple to put these proteins into solution, allow the solution to evaporate, and achieve a large standard crystal.
However, membrane proteins, with several receptors among them, create crystals that are not sufficiently pure and large for typical X-ray crystallography. That said, a large number of these proteins play a role in the development of a disease, which means their structure holds considerable interest to pharmacologists.
But less than 10 years, a solution was eventually identified for membrane proteins. The latest method, known as serial femtosecond X-ray crystallography (SFX), depends on X-ray free-electron lasers that were developed soon before the SFX technique.
What makes it a breakthrough technology is a very high energy density of the laser pulse. The object is exposed to such powerful radiation that it falls apart, inevitably and almost instantly. But before it does, some individual quanta of the laser pulse scatter off the sample and end up at the detector. This is the so-called diffraction-before-destruction principle for studying the structure of the original protein.
Alexey Mishin, Study Co-Author and Deputy Head, Laboratory for Structural Biology of Receptors, MIPT
X-ray free-electron lasers were found to be handy beyond biology: In the last several years, chemists and physicists have increasingly used the SFX technique as well. In 2009, the initial device became available to experimenters, and at present, five centers are open to scientists in the United States, Switzerland, Germany, South Korea, and Japan. While a new device is being developed in China, the U.S. facility—traditionally the first one—has declared plans for upgrades.
The innovative technology has provided scientists a glimpse into the proteins’ structure that earlier eluded analysis, but it has also encouraged new mathematical and technical solutions. In traditional X-ray crystallography, a single crystal is exposed to radiation from numerous angles and the ensuing diffraction patterns are examined collectively.
In the SFX technique, the crystal is immediately damaged by the first interaction with a robust X-ray pulse. Therefore, scientists have to repeat the process with several tiny crystals and examine the resultant “serial” data, thus the name of the technique.
Choosing the samples for SFX is an additional challenge. In traditional X-ray crystallography, it is simply enough to select the highest-quality and largest crystal. This can be performed manually, by eyeing the existing samples. In the latest procedure, a suspension of several tiny crystals of different quality and size needs to be used. Filters and centrifuges with known pore dimensions are used for isolating the crystals by size.
Methods for placing the samples into the chamber also need to be elaborated. X-ray free-electron lasers are known to have a specific maximum frequency at which they can produce radiation pulses. To bring down the cost and time consumption, the chamber should be fed with new crystals at the same frequency.
To date, two methods have been developed to achieve this. Under the first method, the crystals penetrate the chamber in a liquid suspension, provided by an injector. The jet exiting the injector is “squeezed” by a gas stream to make sure that the sample is properly placed. That is, when a crystal is traveling through, it ends up exactly at the core of the laser beam.
On the other hand, the protein crystals can be distributed over a substrate, which is transparent to X-rays, and can be automatically fed into the laser beam before each pulse.
SFX generated its initial results in 2011, and since then, it has exposed more than 200 protein structures. Among these structures are 51 targets that could be significant for pharmacology—viral proteins, ferments, membrane receptors, etc.—that were previously inaccessible to traditional analytical methods.
The systematic analysis of the technology as applied to pharmacology and biology by the MIPT researchers will certainly assist other investigators who are looking to acquire the structures of major drug targets to come up with new kinds of drugs.